Nitrogen stabilizers and fertilizer enhancers are not all equal


Nitrogen is an essential nutrient for plant metabolism, forming the backbone of amino acids, proteins, and chlorophyll, but despite its abundance in the atmosphere, plants often experience nitrogen deficiency due to its limited bioavailability. The nitrogen cycle—driven by soil, microbial, and environmental interactions—regulates nitrogen transformations and losses through processes like volatilization, leaching, and denitrification. To improve nitrogen use efficiency, modern fertilizers and additives have been developed to slow these loss mechanisms, extend nitrogen availability, and support sustainable crop productivity. Earn 0.5 CEUs in Nutrient Management by reading this article and taking the quiz.
Of the 16 essential elements required for plant metabolism, nitrogen is one of the most elusive. One might deliberate: if the atmosphere is approximately 78% nitrogen, then why do so many crops show deficiency symptoms? Why do growers exert so much energy to provide this essential plant food? More importantly, how can we extend the utility of this necessary investment for crop management? To address these questions, we must first address nitrogen’s role in the plant.
Nitrogen, at the very basic level, is combined to the carbon-hydrogen backbone, which forms amino acids, proteins, nucleic acids, nucleotides, coenzymes as well as many other secondary metabolites. Additionally, at the cellular level, nitrogen contributes to chlorophyll synthesis with the help of magnesium. From the human perspective, the plant responds physiologically by becoming greener in color, taller, and healthier and increasing biomass and eventually crop yield compared with a plant that is lacking. Because nitrogen is mobile within the plant, it ensures that critical metabolic needs will be met first. Regardless of the plant type, whether it’s an annual or perennial, it will cannibalize plant structures to reallocate nutrient resources if nitrogen becomes limited. Typically, this is seen as stunted plants or lower leaves exhibiting yellowing or necrotic tissue. Likewise, some perennials will readily abort fruit or leaves.

In situations of overfertilization of available nitrogen, plant responses become more visually obvious. These include lodging, excessive growth, very dark green foliage color, limited crop development, limited reproductive structures, delayed maturity, reduced quality, increased insect or disease incidence, and maintenance of a predominantly vegetative growth habit. Severe excess N could result in root pruning from salts, unforeseen nutrient deficiencies, tissue damage, or even global plant death. Nitrogen also demonstrates significant metabolic relationships with other secondary nutrients (Ca, S), macronutrients (K, P), and micronutrients (Fe, Mn, Mo, Na, and Zn). Diligent attention to soil analysis, tissue analysis, and plant growth patterns should always be considered to maintain critical nutrient ratios and adequate nutrient levels.
Nitrogen uptake, cycling, and losses in soil
Nitrogen uptake through the root system is primarily achieved through two forms: ammonium ions (NH4) and nitrate (NO3). Additional nitrogen forms may include amino acids derived from organic sources such as manure or compost. Ammonium is preferred by plants to efficiently produce proteins, lipids, and various metabolites. These organic compounds are biosynthesized as the ammonium combines with carbon skeletons to form glutamic acid as its base. The nitrogen metabolism process is facilitated through the glutamate synthase and glutamine synthetase enzymes found in the glutamate pathway. Additional pathways will be used by the plant for the nitrates, which are reduced to ammonium N at an energy expense provided by photosynthesis.
The nitrogen cycle encompasses the transformation of nitrogen as it moves through the atmosphere, soil, plants, and soil biomass. Since its recognition in the mid-19th century, the nitrogen cycle has identified organic and inorganic N sources as atmospheric, soil organic matter, N2 fixation, crop residue decomposition, biomass mineralization, clay layer release, and fertilizer additions. This is not a static system. Nitrogen will enter and exit the cycle constantly without human intervention as relatively balanced inputs and outputs. Predominant contributors that perpetuate the cycle include soil water availability, soil chemistry reactions, enzymatic reactions, aeration, temperature, weather, soil macroinvertebrates, and bacterial and fungal activity.
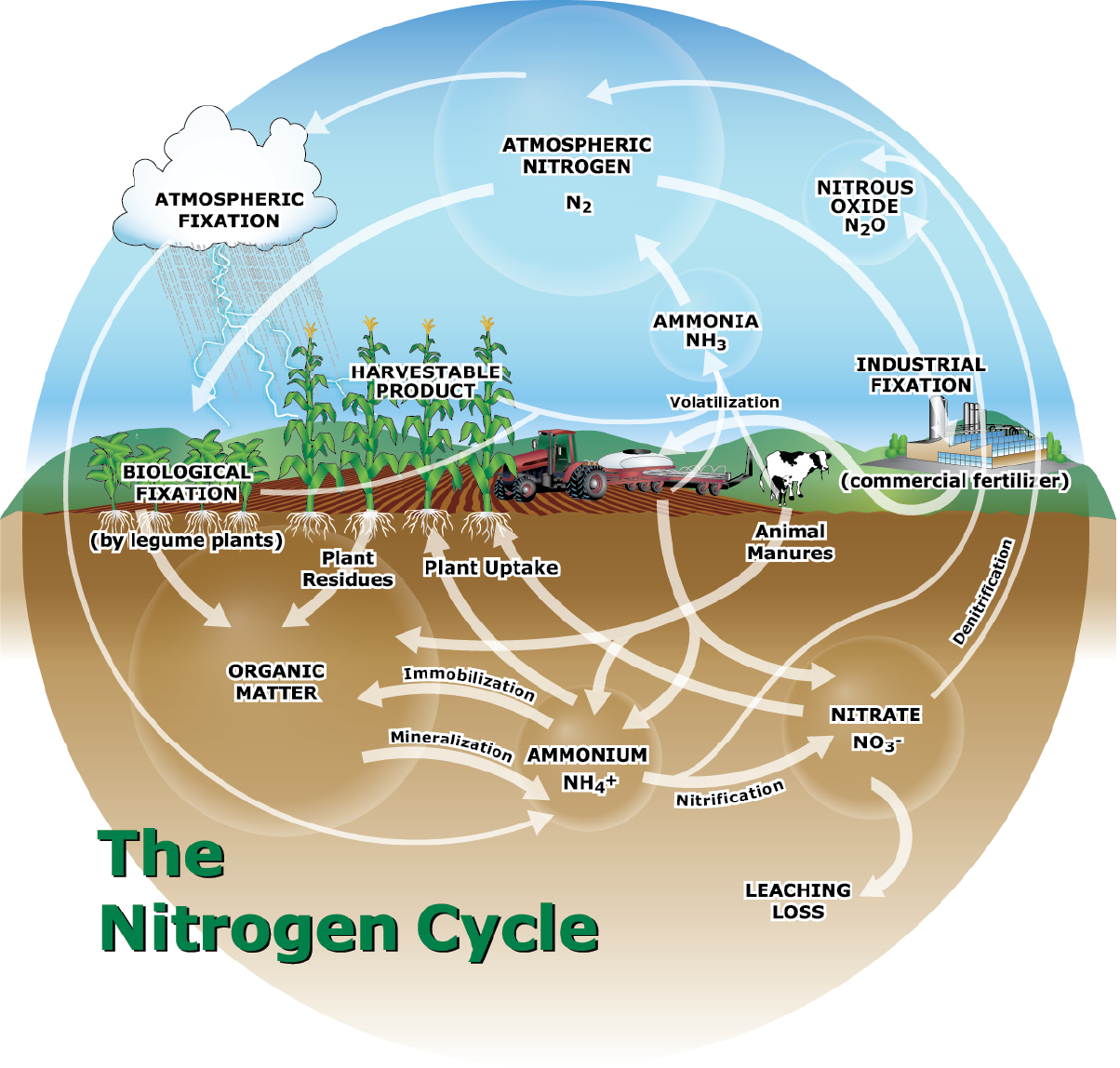
Predominant losses of nitrogen associated with a given land area are determined by the fact that nitrogen can be both mobile and immobile in the soil. Ammonium (NH4) is positively charged (immobile), and conversely, NO3 is negative (mobile). Ammonium (NH4) is subject to chemical, bacterial (Nitrosomonas), and enzymatic activity, which leads to volatilization and nitrification losses. Soil organic matter and specific clay lattices can temporally fix ammonium ions. Nitrates are also subject to chemical, bacterial (Nitrobacter), and enzymatic activity, which leads to denitrification losses. While soil organic matter can temporarily fix nitrates, they are still much more prone to leaching. Losses from excess or diminished soil water and soil erosion affect all nitrogen forms. Eventually, nitrogen losses are due to relentless enzymatic activity as the molecule is very persistent and may originate from multiple sources.
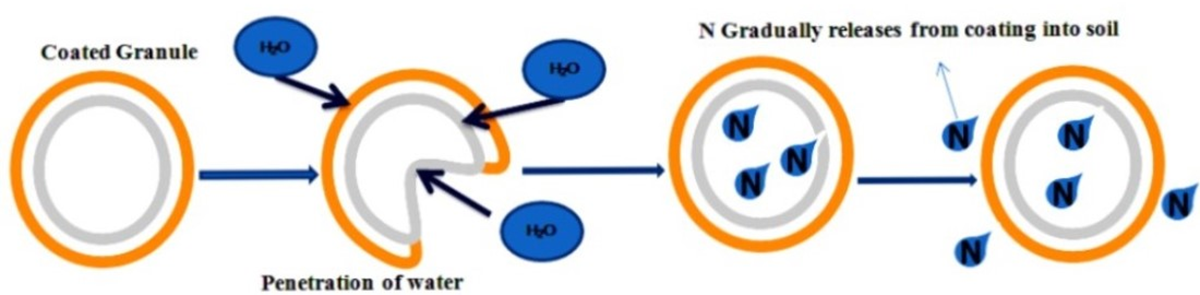
Modern nitrogen fertilizers have been engineered with additives such as sulfates, complex urea compounds, macronutrients, waxes, and polymer coatings to slow loss mechanisms and extend nitrogen availability. The most widely used synthetic nitrogen fertilizer is urea (carbamide), valued for its availability, ease of handling, manufacturing efficiency, concentration, rapid application, and relatively low cost. The primary disadvantage of urea exists in its nitrogen loss avenues. The basic urea prill undergoes rapid hydrolysis by means of the urease enzyme in the presence of water. This pernicious enzyme is prevalent in soil, crop residues, leaf surfaces, and bacterial activity. Once hydrolysis begins, the urea is quickly converted to ammonia and then into ammonium. If the ammonium is not incorporated into the soil but remains on the soil surface, then it will be converted into ammonia gas (NH3), which is lost to the atmosphere. The severity of loss will depend upon soil pH, temperature, and moisture.
Fertilizer additives for nitrogen efficiency
NBPT
NBPT [N-(n-butyl) thiophosphoric triamide] is a urease enzyme inhibitor that reduces urea breakdown by binding to the urease active site. This, in turn, will temporarily decrease ammonia volatilization. This is widely used on dry urea and with UAN (urea ammonium nitrate) solutions. NBPT is best suited for use as surface-applied preplant, topdressed, or sidedressed applications. Many formulations exist with this active ingredient that may contain 12 to 30% active ingredients. NBPT will degrade by oxidative reactions and microbial decomposition. This active ingredient will only stabilize nitrogen from ammonia volatilization and not from other nitrogen loss mechanisms.
Duromide
Duromide is an additive for NBPT to further extend the longevity of the active ingredient. This product will further decrease ammonia volatilization compared with NBPT alone. This is widely used on dry urea and with UAN solutions. NBPT/Duromide is best suited for use as surface-applied preplant, topdressed, or sidedressed applications. This active ingredient combination will only stabilize nitrogen from ammonia volatilization and not from other nitrogen loss mechanisms.
NPPT
NPPT [N-(n-propyl) thiophosphoric triamide] is another partner with NBPT to further extend the longevity of the active ingredient. This additive will further decrease ammonia volatilization compared with NBPT alone. This is widely used on dry urea and with UAN solutions. NBPT/NPPT is best suited for use as surface-applied preplant, topdressed, or sidedressed applications. This active ingredient combination will only stabilize nitrogen from ammonia volatilization and not from other nitrogen loss mechanisms.

The other forms of loss in the nitrogen cycle are nitrification and denitrification. The nitrification process is the microbial conversion of NH4 into NO3. This two-step aerobic process is carried out primarily by Nitrosomonas (NH4 to NO2) and Nitrobacter (NO2 to NO3) bacteria. While nitrate is highly plant-available, again, it is also highly mobile in the soil and more prone to leaching and denitrification losses. Denitrification is the microbial reduction of nitrate into gaseous forms of nitrogen, which escape into the atmosphere. This anaerobic process occurs when soils are saturated or oxygen limited. Denitrification represents a major nitrogen loss pathway in many agricultural soils.
Dicyandiamide
Dicyandiamide (DCD) (2-Cyanoguanidine) is a nitrification inhibitor that blocks the active site of the ammonia monooxygenase enzyme. This will slow the conversion of ammonia to nitrate. This is commonly used with UAN solutions or anhydrous. Dry urea applications are also an application option. Dicyandiamide is best suited for use as below-surface applications. Many formulations exist with this active ingredient that may contain 20 to 30%. Dicyandiamide has been shown to be temporarily detrimental to nonspecific microbial populations. This active ingredient will only stabilize nitrogen from nitrification and denitrification and not from other nitrogen loss mechanisms.
Nitrapyrin

Nitrapyrin [2-chloro-6-(trichloromethyl) pyridine] is a nitrification inhibitor that inhibits the activity of Nitrosomonas bacteria. This will slow the conversion of ammonium to nitrate. This is commonly used with UAN solutions or anhydrous. This active ingredient is best suited for use as below-surface applications. Nitrapyrin will degrade by microbial decomposition within 30 days. Nitrapyrin has been shown to have short-term effects on nonspecific microbial populations. This active ingredient will only stabilize nitrogen from nitrification and denitrification and not from other nitrogen loss mechanisms.
Pronitridine
Pronitridine [(N″-cyanoguanidine) pyridine] is a nitrification inhibitor that inhibits the activity site of the ammonia monooxygenase enzyme in the Nitrosomonas bacteria. This will slow the conversion of ammonia to nitrate. This is commonly used with UAN solutions or anhydrous ammonia. This active ingredient is best suited for use as below-surface applications and will only stabilize nitrogen from nitrification and denitrification and not from other nitrogen loss mechanisms.
Polymer exchange resins
Polymer exchange resins are additional additives that may be included with NBPT or DCD-type active ingredients. The purpose is to provide additional benefits by extending the longevity of the specific active ingredient. The exchange resin is built with a robust cation exchange capacity that will complex with the ammonium ions. The ammonium is eventually released for plant uptake by exchanging the nutrient for hydrogen ions. Hence, there is a microenvironment of low pH surrounding the treated nitrogen. This prevents immediate biological activity, which could lead to losses. The resins are also formulated to penetrate the interior of the fertilizer prill to offer comprehensive coverage of the applied active ingredients.
Several other nitrogen stabilizers are currently under investigation by researchers. Those have shown improvements in further preventing nitrogen losses. However, the newer actives are not currently registered in the U.S.
Crop inputs and methodologies must be timely and precise. Agronomically sound practices of nitrogen management should continue to include the four Rs. The “right source” involves many options, all of which have advantages and disadvantages. The sole purpose of accurate nitrogen rates is to supply plant needs for peak crop performance and not cause excess nitrogen losses. Nitrogen applications that are incorporated in the soil as ammonium or urea forms are still subject to losses. Additional approaches can be incorporated to extend or preserve nitrogen applications due to inevitable losses. Table 1 summarizes common nitrogen sources and their relative attributes for stability, solubility, and release characteristics.
| AMS/ATS | UAN/CAN-17 | SCU | Hard polymer/ wax coatings | Urea-formaldehyde (methylene diurea) | Urea-triazone | IBDU |
|---|---|---|---|---|---|---|
| Stable form | Fast plant response | Great for turf and ornamental | Relatively stable form | Stable form | Relatively stable liquid form | Stable form of urea |
| Very soluble | Very soluble | Slow release from wax | Slow release from polymer | Relatively slow release from chemical conversion | Slow release from chemical conversion | Low solubility |
| Easy to use | Easy to use | Easy to use | Easy to use | Specialty purpose | Specialty purpose | Specialty purpose |
| Limited rate use | Availability varies | Limited to small application areas | Temperature & moisture dependent | Temperature & bacterial dependent | Limited rate use foliar | Slow release from chemical conversion |
| Hydrophilic | Corrosive to metals | Can be pricey | Availability varies | Availability varies | Availability varies | Availability varies |
| Subject to N losses | Subject to N losses | Subject to N losses | Subject to N losses | Subject to N losses | Subject to N losses | Subject to N losses |
| Blending issues | Coating has longer breakdown | Can be pricey |
Note: AMS, ammonium sulfate; ATS, ammonium thiosulfate; UAN, urea ammonium nitrate; CAN-17, calcium ammonium nitrate; SCU, sulfur-coated urea; IBDU, isobutylidenediurea.
Note: The commercial products listed here are for educational purposes only and not intended for promotion or endorsement.
Adotey, N., McClure, A., & Yin, X. (2020). Enhanced efficiency nitrogen fertilizer as a tool to control nitrogen loss in row crop production (PB 1888). University of Tennessee Extension.
Banerjee, S., & Aggarwal, A. (2013). Potential clinical significance of urease enzyme. European Scientific Journal, 9(21).
Goos, R. J. (2019). Evaluation of two products recently introduced as nitrification inhibitors. Communications in Soil Science and Plant Analysis, 50(5), 503–511. https://doi.org/10.1080/00103624.2019.1566466
Johnson, C., Albrecht, G., Ketterings, Q., Beckman, J., & Stockin, K. (2005). Nitrogen basics–the nitrogen cycle. Agronomy Fact Sheet Series, Fact Sheet 2. Cornell University Cooperative Extension Service.
Lan, T., Chen, X., Liu, S., Zhou, M., & Gao, X. (2023). Biological and chemical nitrification inhibitors exhibited different effects on soil gross N nitrification rate and N₂O production: a ¹⁵N microcosm study. Environmental Science and Pollution Research, 30, 116162–116174. https://doi.org/10.1007/s11356-023-30638-x
O’Callaghan, M., Gerard, E. M., Carter, P. E., Lardner, R., Sarathchandra, U., Burch, G., Ghani, A., & Bell, N. (2010). Effect of the nitrification inhibitor dicyandiamide (DCD) on microbial communities in a pasture soil amended with bovine urine. Soil Biology and Biochemistry, 42(9), 1425–1436.
Qiu, X.-M., Sun, Y.-Y., Ye, X.-Y., & Li, Z.-G. (2020). Signaling role of glutamate in plants. Frontiers in Plant Science, 10, 1743. https://doi.org/10.3389/fpls.2019.01743
Ren, B., Ma, Z., Zhao, B., Liu, P., & Zhang, J. (2022). Nitrapyrin mitigates nitrous oxide emissions and improves maize yield and nitrogen efficiency under waterlogged field. Plants (Basel), 11(15), 1983. https://doi.org/10.3390/plants11151983
Thapa, R., Chatterjee, A., Awale, R., McGranahan, D. A., & Daigh, A. (2016). Effect of enhanced efficiency fertilizers on nitrous oxide emissions and crop yields: A meta-analysis. Soil Science Society of America Journal, 80, 1121–1134. https://doi.org/10.2136/sssaj2016.06.0179
Tisdale, S. L., & Nelson, W. L. (1975). Soil Fertility and Fertilizers (3rd ed.). Macmillan, New York.
Xiang, Y., Ru, X., Shi, J., Song, J., Zhao, H., Liu, Y., & Zhao, G. (2018). Granular, slow-release fertilizer from urea–formaldehyde, ammonium polyphosphate, and amorphous silica gel: A new strategy using cold extrusion. Journal of Agricultural and Food Chemistry, 66, 7606–7615.
Zayed, O., Hewedy, O. A., Abdelmoteleb, A., Ali, M., Youssef, M. S., Roumia, A. F., Seymour, D., & Yuan, Z.-C. (2023). Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules, 13, 1443. https://doi.org/10.3390/biom13101443
Self-study CEU quiz
Earn 0.5 CEUs in Nutrient Management by taking the quiz for the article. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
Once nitrogen enters the plant, it is converted to various amino acids, proteins, nucleotides, etc. The plant also will synthesize
a. secondary metabolites.
b. chlorophyll.
c. both a and b.
d. none of the above.
Ammonium forms of nitrogen are generally more stable in soil than nitrate forms. Which enzyme’s active site is blocked by a nitrification inhibitor?
a. Acid phosphatase.
b. Urease.
c. Ammonia monooxygenase.
d. Nitrogenase.
Which obligate autotrophic bacterial genus initiates the nitrification process by converting NH4 to NO2?
a. Nitrosomonas.
b. Nitrobacter.
c. Bacillus.
d. Pseudomonas.
4. Since urea is prone to nitrogen losses, which of the following can help extend its longevity in soil and improve nitrogen use efficiency?
a. Polymer or resin coatings.
b. Urease inhibitors such as NBPT.
c. Controlled-release formulations.
d. All of the above.
5. Dicyandiamide (DCD), when used with dry or UAN forms of nitrogen, prevents all forms of nitrogen loss.
a. True.
b. False.
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.










