The influence of nozzle type and application speed on sicklepod control with cadre and butyrac

Sicklepod, also colloquially known as coffeeweed, is one of the most common and troublesome weeds in Georgia peanut production systems. Spray chamber/greenhouse studies were conducted to evaluate the effects of application speed (8 and 12 MPH), nozzle type (XR, TTI, and AIXR), and herbicide treatment on sicklepod control.

Sicklepod [Senna obtusifolia (L.) H.S. Irwin & Barneby], also colloquially known as coffeeweed, is one of the most common and troublesome weeds in Georgia peanut production systems (Figures 1 and 2). In fact, a 2019 survey of more than 1,700 Georgia growers indicated that sicklepod was the fifth most challenging of all agricultural pests, including weeds, insects, and diseases (Culpepper et al., 2020). Sicklepod has also been reported to be a problematic weed in many other states, including Alabama, Arkansas, Florida, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, Texas, and Virginia (Teem et al., 1980).
Sicklepod can be very difficult to control in peanut partly because they are both members of the same plant family (Fabaceae). Thus, sicklepod is tolerant of many of the herbicides used for weed control in peanut. Additionally, sicklepod can germinate/emerge under a wide range of environmental conditions and produce large amounts of seed (up to 14,000 seed/plant) that persist in the soil seedbank (Boza et al., 1989; Egley & Chandler, 1978). From a competition viewpoint, sicklepod populations of 6.7 plants/33 row ft have reduced peanut yields by as much as 27% (Barbour & Bridges, 1995).
Current control strategies for sicklepod in peanut are based upon the use of narrow row spacing and herbicides. Previous research has shown that sicklepod control was 9% greater when peanut was seeded in a twin-row pattern compared with a single-row pattern (Lanier et al., 2004). The two mostly commonly used postemergence herbicides for the control of sicklepod in peanut are Cadre 2AS (imazapic) and Butyrac 175 (2,4-DB). According to a recent USDA-NASS survey, these herbicide active ingredients are used on 59 and 34% of the peanut acres in Georgia (USDA-NASS, 2019). Frequently, these herbicides are tank-mixed together to broaden the spectrum of control.
In recent years, some growers have anecdotally observed less control of sicklepod with Cadre and/or 2,4-DB. Potential causes for this perceived reduction in sicklepod control could be related to many factors including environmental conditions, weed height/stage of growth, application speed, and nozzle type. Cadre resistance, or more specifically, ALS resistance, in Georgia sicklepod populations has not yet been identified (Carter, 2018). Potential sicklepod resistance to Butyrac has not been investigated.
New Research

Spray chamber/greenhouse studies (Figure 3) were conducted at the Pesticide Application Technology Laboratory located at the West Central Research and Extension Center in North Platte, NE in 2019 to evaluate the effects of application speed (8 and 12 MPH), nozzle type (XR, TTI, and AIXR), and herbicide treatment on sicklepod control. Herbicide treatments included Cadre 2AS at 4 oz/ac + COC (R.O.C) at 1% v/v, Butyrac 1.75SL at 24 oz/ac + COC at 1% v/v, and Cadre 2AS at 4 oz/ac + Butyrac 1.75SL at 24 oz/ac + COC @ 1% v/v.
The study was arranged as a randomized complete block design with factorial arrangement of treatments with five replications and two independent experimental runs. Postemergence herbicide applications were made using a three-nozzle research track sprayer with nozzles spaced 20 inches apart and 20 inches above the plants applied at 15 GPA and at 40 PSI to sicklepod plants that were either 3 or 6 inches tall. Nozzles with the same spray angle (110°) but different orifice sizes (04 and 06) were used to keep the application volume consistent when changing the application speed.
Aboveground dry weight biomass reductions were calculated at 28 days after treatment (DAT). Data were subjected to ANOVA using a generalized linear mixed model (PROC GLIMMIX) in SAS (Statistical Analysis Software, version 9.4, Cary, NC) with mean separations made at the α = 0.05 level using Fisher’s protected LSD test and the Tukey adjustment. Data were pooled over experimental runs.
Results and Discussion
There were no interactions among main effects of herbicide, nozzle type, and application speed; thus, data are presented by main effects.
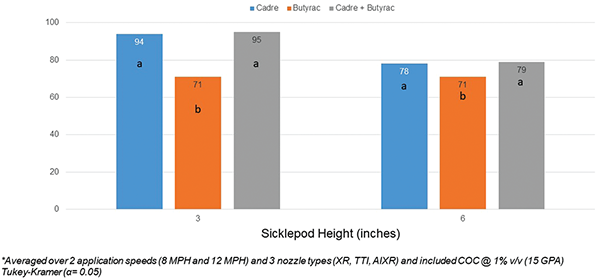
When averaged over application speed and nozzle type, Cadre reduced sicklepod biomass by 94% when applied at 3 inches and 78% when applied at 6 inches (Figure 4). Butyrac tank mixtures with Cadre did not significantly improve sicklepod biomass reductions. In more than 50 field trials conducted in the Southeast, postemergence applications of Cadre provided 86% control of sicklepod (Grey et al., 2003). Sicklepod biomass was reduced 71% with Butyrac alone, regardless of height. In prior peanut field research, 2,4-DB provided 69% control of sicklepod (Lancaster et al., 2005).
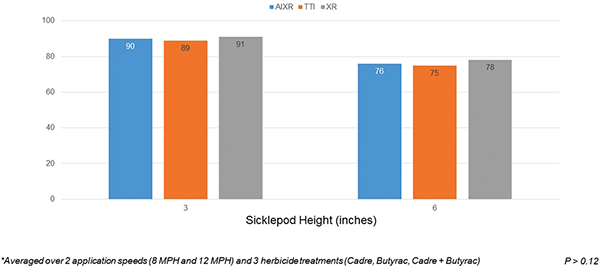
When averaged over application speed and herbicide treatment, nozzle type had no effect on sicklepod biomass reduction (Figure 5). Nozzle type did not influence Palmer amaranth (Amaranthus palmeri S. Watson) control in other peanut weed control research (Carter et al., 2017). Additionally, Palmer amaranth control with Cobra (lactofen) was not influenced by nozzle type (Berger et al., 2014). However, nozzles that produce coarser droplets have been reported to reduce the control of many weed species in other studies (Carter et al., 2017; Meyer et al., 2016). Optimum nozzle type, water carrier volume, and spray pressure is herbicide and weed species specific (Sikkema et al., 2008).
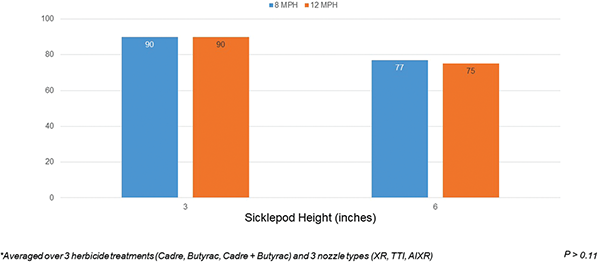
When averaged over herbicide treatment and nozzle type, application speed had no effect on sicklepod biomass reduction (Figure 6). Previous research has shown that application speeds of 5, 10, or 15 MPH did not influence weed control with a combination of Clarity (dicamba) + Roundup PowerMax (glyphosate) (Rodrigues et al., 2018). In contrast, sprayer speed was highly negatively correlated with spray coverage, which in theory, could reduce weed control (Nansen et al., 2015).
Summary
- Sicklepod can be a difficult weed to control in peanut.
- Sicklepod height at the time of application is critical for optimum postemergence control.
- Cadre is the most effective postemergence herbicide for the control of sicklepod in peanut.
- Nozzle type and application speed did not influence the control of sicklepod when applying Cadre and/or Butyrac.
Dig deeper
Barbour, J.C., & Bridges, D.C. (1995). A model of competition for light between peanut (Arachis hypogaea) and broadleaf weeds. Weed Science43, 247–257.
Berger, S.T., Dobrow, M.H., Ferrell, J.A., & Webster, T.M. (2014). Influence of carrier volume and nozzle selection on Palmer amaranth control. Peanut Science41, 120–123.
Boza, R.C., Oliver, L.R., & Driver, T.L. (1989). Intraspecific and interspecific sicklepod (Cassia obtusifolia) interference. Weed Science37, 670–673.
Carter, O.W. (2018). Addressing the current chemical weed control challenges in Georgia peanut production. Ph.D. dissertation, Department of Crop & Soil Sciences, The University of Georgia). Retrieved from https://bit.ly/2VtQ4CJ.
Carter, O.W., Prostko, E.P., & Davis, J.W. (2017). The influence of nozzle type on peanut weed control programs. Peanut Science44, 93–99.
Culpepper, A.S., Vance, J.C., Gray, T., Johnson, L.P., & Prostko, E.P. (2020). Using pesticides wisely—2019. Proceedings of the Weed Science Society of America. New Orleans, LA: Weed Science Society of America.
Egley, G.H., & Chandler, J.M. (1978). Germination and viability of weed seed after 2.5 years in a 5-year buried seed study. Weed Science26, 230–239.
Grey, T.L., Bridges, D.C., Prostko, E.P., Eastin, E.F., Johnson, W.C. III, Vencill, W.K., … Wilcut, J.W. (2003). Residual weed control with imazapic, diclosulam, and flumioxazin in southeastern peanut (Arachis hypogaea). Peanut Science30, 22–27.
Lancaster, S.H., Jordan, D.L., Spears, J.F., York, A.C., Wilcut, J.W., Monks, D.W., Batts, R.B., & Brandenburg, R.L. (2005). Sicklepod (Senna obtusifolia) control and seed production after 2,4-DB applied alone and with fungicides or insecticides. Weed Technology19, 451–455.
Lanier, J.E., Lancaster, S.H., Jordan, D.L., Johnson, P.D., Spears, J.F., Wells, R., Hart, C.A., & Brandenburg R.L. (2004). Sicklepod control in peanut seeded in single and twin row patterns. Peanut Science31, 36–40.
Meyer, C.J., Norsworthy, J.K., Kruger, G.R., & Barber, T.L. (2016). Effect of nozzle selection and spray tank volume on droplet size and efficacy of Engenia tank-mix combinations. Weed Technology30, 377–390.
Nansen, C., Ferguson, J.C., Moore, J., Groves, L., Emery, R., Garel, N., & Hewitt, A. (2015). Optimizing pesticide spray coverage using a novel web and smartphone tool, SnapCard. Agronomy for Sustainable Development35, 1075–1085.
Rodrigues, A.O., L. G. Campos, C. F. Creech, B. K. Fritz, U. R. Antuniassi, and G. R.Kruger. (2018). Influence of nozzle type, speed, and pressure on droplet size and weed control from glyphosate, dicamba, and glyphosate plus dicamba. In B. Fritz & T. Butts (Eds.) Pesticide formulation and delivery systems: Innovative application, formulation, and adjuvant technologies (Vol. 38, pp. 61–75). West Conshohocken, PA: ASTM International. https://doi.org/10.1520/STP161020170249Sikkema, P.H., Brown, L., Shropshire, C., Spieser, H., & Soltani, N. (2008). Flat fan and air induction nozzles affect soybean herbicide efficacy. Weed Biology and Management8, 31–38.
Teem, D.H., Hoveland, C.S., & Buchanan, G.A. (1980). Sicklepod (Cassia obtusifolia) and coffee senna (Cassia occidentalis) geographic distribution, germination, and emergence. Weed Science28, 68–71.
USDA-NASS. (2019). USDA–NASS Chemical Use Surveys—Peanut. Retrieved from https://bit.ly/2Vvt4Uc.
Olumide S. Daramola, Gregory E. MacDonald, Ramdas G. Kanissery, Barry L. Tillman, Hardeep Singh, Oluseyi Ayodeji Ajani, Pratap Devkota, Implications of planting date on Benghal dayflower ( Commelina benghalensis L.) and sicklepod ( Senna obtusifolia L.) management in peanut , Weed Technology, 10.1017/wet.2024.50, 38, (2024).
Olumide S. Daramola, Gregory E. MacDonald, Ramdas G. Kanissery, Barry L. Tillman, Hardeep Singh, Pratap Devkota, Influence of carrier water pH and hardness on imazapic efficacy for sicklepod ( Senna obtusifolia L.) control in peanut , Weed Technology, 10.1017/wet.2023.96, 38, (2024).
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.