The ‘essence’ of genomic selection
We started using DNA fingerprints to select for new crop varieties more than 30 years ago. What have we learned, and what’s coming next?

- Crop production is a science that needs to constantly evolve in order for farmers to keep producing high yields in spite of emerging agricultural challenges. One strategy to enhance crop-breeding programs, genomic selection, allows breeders to predict the traits of a crop based on its DNA, before it’s ever grown.
- While it took some time for genomic selection to be widely adopted in breeding programs, it now is an essential tool for many researchers, cutting down the time, labor, and resources needed to produce new crop lines.
- The future of genomic selection will likely see breeders incorporating new technologies, like recent breakthroughs in AI, into their programs. Genomic selection’s applications and prospects were recently reviewed in The Plant Genome journal.
At any point during the growing season, a crop will run into some kind of stress—plant diseases, nutrient deficiencies, drought, and hungry pests. How do farmers make sure their crops are well defended against environmental stressors, produce high yields, and are nutritious, for a global population of soon-to-be 10 billion people?
Humans have been breeding plants ever since we started farming. From teosinte came corn, from wild emmer came wheat—virtually every crop we eat today has been drastically changed by people, selecting for food with big grains, high yields, and tasty fruits. Generations of breeding have brought up higher and higher yielding crops. But with modern agricultural challenges, we have begun to also prioritize crops that can withstand the most stressors without compromising taste and profit.
“You're working towards everything,” says Diana Escamilla, plant-breeding scientist at Avalo. “You're trying to enhance the yield and quality of crops, increasing disease resistance and ensuring climate resilience—this all requires understanding of crop physiology and how plants respond to weather. This field allows me to get the big picture of crop production.”

But there’s a catch: plant breeding is hard, and it takes time and resources to do properly.
“Traditional plant breeding requires growing several successive generations of plants while selecting for the best, most fit crop,” Escamilla explains. “And selecting based off of phenotypes (visible traits) requires having plants in the field to properly observe those traits. Phenotyping thus costs a lot of resources: labor and money. Breeders are often limited by a budget, so [historically] they could not evaluate as many individuals as they would like.”
In order to keep up with demand, plant breeding is a constantly innovating field, adopting new technologies and tools to be faster and more precise. A recent review in The Plant Genome led by Escamilla, then postdoctoral research associate at Iowa State University, details one such tool that became a key part of many global breeding programs—genomic selection.

Genomic selection uses the information from a plant's genes and its matching phenotypes to predict the traits of new, untested plants. Rex Bernardo, Professor and Endowed Chair of Corn Breeding and Genetics at the University of Minnesota, describes genomic selection as a “quick and cheap way to evaluate plants based on DNA fingerprints.”
The history of genomic selection
While it may be hard to believe now, with how widespread genomic selection (GS) is currently used in breeding programs, it took years for the technique to become so ubiquitous. Developing genomic selection was a decades-long process; it was years of adding to and expanding upon other’s scientific findings.
Jianming Yu, co-author of The Plant Genome review, notes how molecular markers—molecules that differentiate different species, or different individuals within a species—were being used for marker-assisted selection in the early 1990s. Breeders found that mapping and cloning the gene that makes up a QTL (quantitative trait locus, i.e., many genes that determine a specific trait) could lead to breeding guided by specific genes.
"That was tremendously successful, but only for the major-effect QTLs, and not for complex traits," explains Yu, Professor and Pioneer Distinguished Chair in Maize Breeding at Iowa State University. "Because complex traits are controlled by multiple genes and their interactions, isolating individual genes seemed to be a lengthy process. Gradually, people became disappointed, saying that molecular markers were not as helpful in large-scale plant breeding as we had hoped for.”
Over the next decade, Rex Bernardo notes that GS became better known because of a 2001 publication in Genetics by animal scientists Theo Meuwissen, Ben Hayes, and Mike Goddard.
“That paper opened the floodgates to the possibility of predicting the performance of individuals that have been genotyped from information about the performance of individuals that had been both genotyped and phenotyped,” he says.
The work by Meuwissen and colleagues is considered by many to be one of the first to conclude that genotypic values can be predicted from markers with high success. Bernardo says that in this 2001 paper, the researchers determined effects each molecular marker in an individual has on a trait, like yield. But he adds that after the publication of this paper, another method resurfaced he describes as “more computationally efficient.” Instead of calculating marker effects, it used markers to estimate the relatedness among individuals.
The four steps to genomic selection
Phenotyping is given a new role in genomic selection: instead of directly observing traits and advancing selection through several generations of plants, breeders use the known phenotypes and genotypes of parent plants to generate data for models. These models will later be used to predict the traits of plants with known genotypes, but unknown phenotypes.
Genomic selection can be thought of as four steps: Designating the “starter” population, building a model based off of this population, predicting traits, and selecting plants that have desirable traits.
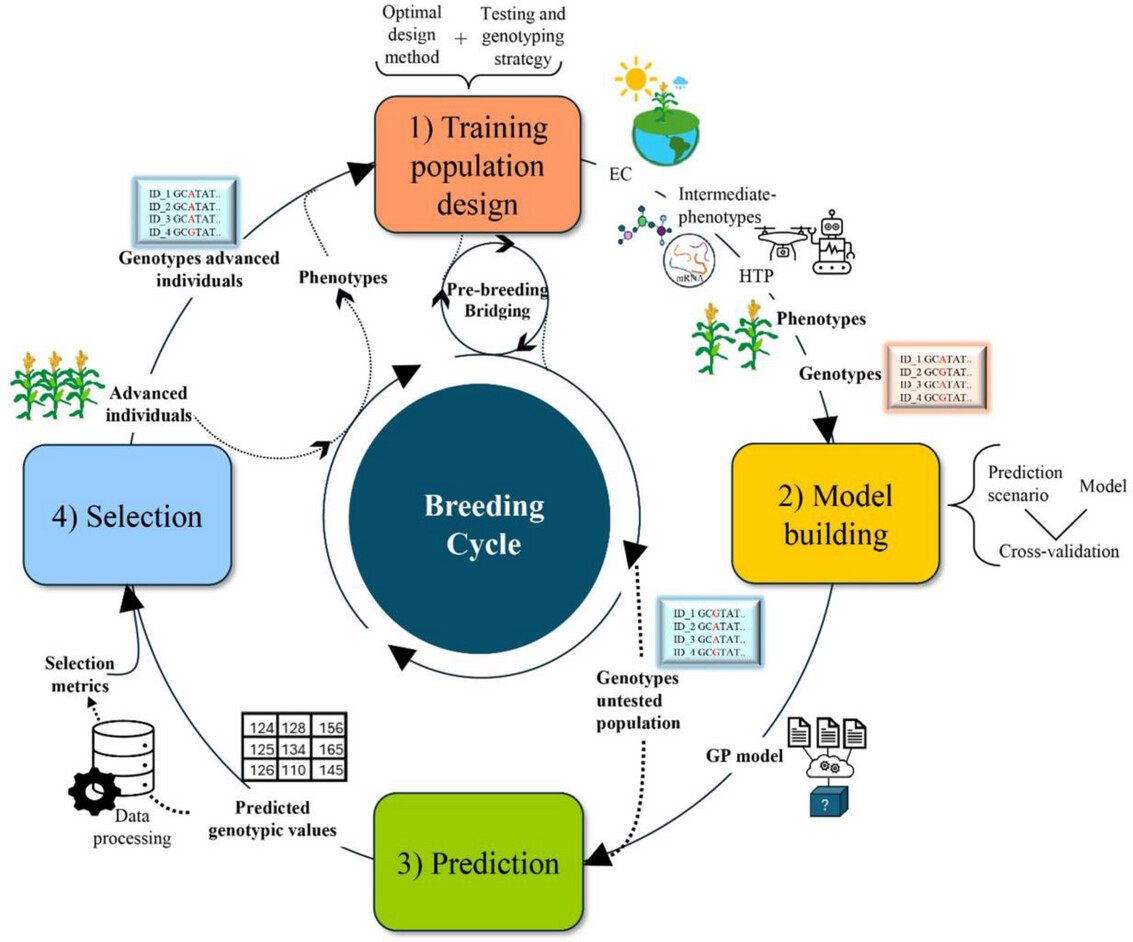
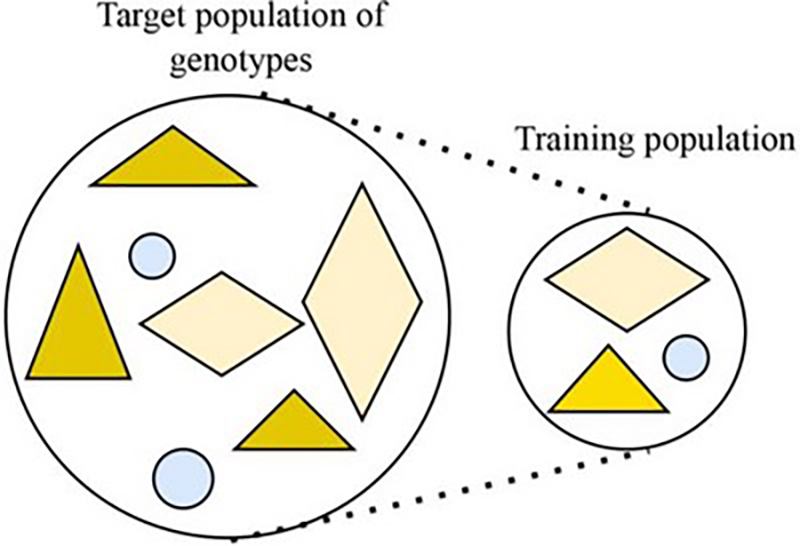
First, breeders select the initial plants they want to use to train their model. From this group, the breeders will want to enhance a trait. Maybe the plants the breeder is working with are from a species that can produce different colored flowers, and they want the new line to only produce purple flowers. It isn’t possible to train a model using all possible plants at their disposal, so breeders will choose a subsection of these plants to start with. Whether breeders are starting completely from scratch or have data from past breeding programs, they want to make sure the plants in their training population represent the initial population’s genetic diversity.
“What you want in a training population is a population that captures all of the diversity that you observe in your [initial] population, Escamilla explains. “So, if I’m working with plants that can have either purple, white, or yellow flowers, I try to have [at least] one representative of each flower color.”
Once the training population has been established, then you can build your model. Any known genotypic and phenotypic information you have can be included. The model correlates known genetic data with known phenotypic data from all individuals in your training population.
Once those correlations are established, you can then predict the likelihood of individuals with unknown phenotypic data to have certain traits, based on what’s in their DNA—this is also known as their genotypic value.
The “best” individuals (with the highest predicted genotypic values) are chosen to be used as parents for the next generation. Individuals with low predicted values are removed. And then the cycle continues, advancing a population until you have all your desired traits (which could be flower color, but could also be yield, stress resilience, fiber strength, flavor quality—any trait with a strong genetic influence). Since genomic selection allows breeders to make predictions and select to advance without the physical need to grow as many plants as before, this saves time and money.
“So, for example, we can evaluate the milk yield of a dairy bull,” Bernardo says. “And you'd say, ‘Well, what's the milk yield of a dairy bull?’ Well, it's zero. Bulls don't produce milk. But a bull has genes that affect milk performance that it passes on to its female progeny. So, we can assess the potential ‘milk yield’ of a dairy bull based on the milk yield of its mother, half-sister, female first cousin, aunt, and so on. We weigh that information based on the level of relatedness, meaning more information is extracted from the mother because it's more closely related than the half-sister. We've traditionally done this by pedigree information, but we can now also do that by marker information.”
This marker-based procedure, which is now considered to be part of genomic selection, was developed by Bernardo and originally published in 1994 in Crop Science.
After the 2001 Genetics paper was published, it still took a while for crop breeders to adopt genomic selection widely, Yu notes, until a 2007 Crop Science paper by Rex Bernardo (Yu’s Ph.D. adviser) and Yu.
“Even after I did my postdoc at Cornell and started a faculty position at K-State, I still kept talking to Rex,” Yu recalls. “I said if we want researchers to utilize molecular markers in plant breeding, we have to do it—it’s us. Basically, we changed our attitude from saying that genomics may or may not be helpful to saying that we have to make it helpful because of new technology.”
“I said if we want researchers to utilize molecular markers in plant breeding, we have to do it—it’s us. Basically, we changed our attitude from saying that genomics may or may not be helpful to saying that we have to make it helpful...”
Yu attributes the success of genomic selection to new technologies that accelerated plant breeding. Rapid improvements in genotyping and sequencing made it possible to obtain molecular marker data for a large number of individuals, and analytical methods were ready. At the time, major maize industries and biotech companies were transitioning from the traditional technique of selfing-selfing (self-pollinating corn plants for several generations and conducting selection along the way) to the double haploid technique (crossing plants with a haploid inducer to produce kernels with only one set of unpaired chromosomes and then chemically treating these kernels to double their DNA).

“The difference between the two is that with the double haploid technique, suddenly you skip years and generations of selfing,” Yu explains, emphasizing that because of this missing generational advancement, breeders do not have the time to select for desirable traits. “Most of the double haploids made are not going to be part of successful, elite lines. So, we have to apply a selection standard to get rid of many of them. By coupling double haploid technology with this concept of genomic selection, this allowed companies to adopt genomic selection quickly.”
Two years after the 2007 Crop Science paper, another group of leading scientists at Cornell—Elliot Heffner, Mark Sorrells, and Jean-Luc Jannink—published a review and interpretation paper in Crop Science, promoting that genomic selection can make big progress in crop breeding, Yu notes.

“I think it's through these papers, together, that spurred the public and private sectors to start adopting and exploring the methodology in genomic selection. And then once more plant-breeding programs wanted to do genomic selection too, we had to train graduate students. … Now, the students—trained with new concepts, new methodology, and new skills—are hired by companies to push forward their own private efforts.”
While these papers stand out, both Yu and Bernardo note that the development of GS is thanks to the work of many different research teams over the years.
Genomic selection today
Nowadays, genomic selection is used in an extensive number of animal- and crop-breeding programs. Both public and private breeding programs have been able to shorten breeding cycles, improve resource allocation, and increase desired traits in new crop and animal breeds.
Escamilla regularly uses these concepts in her new position. “I was actually talking about this to my former adviser in Iowa, Dr. Yu,” she adds. “We did this review, and then afterwards I joined this company as a field breeding specialist. In this position, I'm designing one of our breeding programs using genomic selection. And I think writing this review has really helped me to balance the trade-offs faced when designing this plant breeding program.”

Ongoing research in genomic selection is essential for improving a breeding programs’ ability to tackle emerging challenges in agriculture. Many agricultural challenges are exacerbated by a gradually warming climate. And that problem isn’t going to go away—temperatures are expected to rise 1.4–2.5°C by 2050, which could lower global crop yields from anywhere between 7–23%, according to a study published in Nature Reviews. But the global demand for food is only getting higher.
One challenge brought on by climate change is increased heat stress. “We want to find individuals that can adapt better to heat and drought,” Escamilla explains. “[But traditional] phenotyping is almost impossible to do at a large scale—there are too many time points to measure, and sometimes you need physical samples to measure traits, which requires destructive sampling. It’s really not that easy to do."

Genomic selection can help with this because of its ability to integrate technologies and tools, Escamilla says.
"For example, GS can be integrated with high-throughput phenotyping. Instead of measuring traits manually, which requires a lot of labor, you can take images of crops and use those images to measure traits and crop development. We can input that data into our GS models. Being able to have models that can handle all this information and make good use of it is the difference between the past and now. The initial GS models have always been evolving to capture more information and to be better at prediction.”
The future of genomic selection
Co-authors Escamilla and Yu are excited by this technological advancement. They noted that other tools beyond high-throughout phenotyping can be integrated with GS, too.
"Genomic selection is evolving, but the essence [remains]. We want to predict phenotypes, but how we do it is what is changing, and it's changing for good.”
Escamilla, who currently works at Avalo, a “nature-based, AI-powered, rapid evolution platform,” is understandably excited by how AI can be integrated with genomic selection. “It could be RNA, proteins, or metabolites—new machine-learning models allow us to input more information, so we can become more precise, Escamilla explains. “So genomic selection is evolving, but the essence [remains]. We want to predict phenotypes, but how we do it is what is changing, and it's changing for good.”
Yu expects new technologies—that collect large quantities of data, edit plant genes, accelerate plant development (speed breeding), and even incorporate wild plants with elite traits into existing breeding programs (de novo domestication)—to be integrated with genomic selection in the next chapter of plant breeding’s history.
“Many years ago, when I was a graduate student in plant breeding, I was questioning why we have to learn all these new things. But now I'm totally changed. ... We need to just search and try out new ways and new ideas to make progress. We need new technology, new analytics, and new design to solve the long-term, long-standing questions in breeding.”
Dig deeper
View the review in The Plant Genome:
Escamilla, D. M., Li, D., Negus, K. L., Kappelmann, K. L., Kusmec, A., Vanous, A. E., Schnable, P. S., Li, X., & Yu, J. (2025). Genomic selection: Essence, applications, and prospects. The Plant Genome, 18, e70053. https://doi.org/10.1002/tpg2.70053
Listen to episode of the podcast Field, Lab, Earth to hear Jianming Yu talk more about genomic selection:
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.









