It’s All Hands on Deck in the Battle Against Herbicide- Resistant Weeds


While we know herbicide resistance is an inevitable fate in a system that relies heavily on herbicides, we can work together to focus on long-term strategies to delay its development and spread. Managing for a single year is a key reason why we are in the situation we are in today. A review of how herbicide resistance issues developed over time provides some insight into this and also some guidance into how we can more effectively deal with the problems we face now and into the future. Farmers rely heavily on their advisers to provide sound agronomic recommendations, and we, as advisers, need to take that role seriously to broach the subject of long-term weed management beyond individual-year herbicide recommendations. Earn 1.5 CEUs in Integrated Pest Management by reading this article and taking the quiz.
Herbicides have played a key role in weed management since the introduction of the first synthetic herbicides in the late 1940s (Shaner, 2014). Unfortunately, their use also selects for herbicide‐resistant (HR) weed populations. This conundrum has led to increasingly complex weed management challenges for farmers across the globe.
Herbicides can be categorized by their mode of action (the biological process affected by the herbicide) and their site of action (the specific biochemical site affected by the herbicide). The Take Action “Herbicide Classification” chart (see Resources) is a useful tool that summarizes commonly used herbicides by their site of action as indicated by their herbicide group number. An herbicide group number is a specific subset of an herbicide mode of action. This system was developed in part to aid in the management of HR weeds.
A review of how herbicide resistance issues developed over time provides some insight into how we got into the situation we are in today and also some guidance into how we can more effectively deal with the problems we face now and into the future.
Herbicide Resistance Is Not a New Phenomenon
Herbicide resistance was first reported in wild carrot and climbing dayflower with 2,4‐D, a Group 4 (synthetic auxin) herbicide in 1957, but HR cases remained rare until the 1970s when broadleaf weeds resistant to triazine (Group 5 herbicides), also known as the photosystem II inhibitors (e.g. atrazine), were reported (Heap, 2023). The overall consensus was that herbicide resistance was manageable since resistance to the Group 5 herbicides incurred a significant fitness cost and other herbicide options were available to control HR weeds. Although resistance to a Group 3 (microtubule assembly inhibitor) herbicide was reported in 1973, resistance to the Group 5 herbicides was by far the dominating factor driving the number of unique HR weed cases (species by site of action) reported globally from 3 in 1970 to 38 in 1980, an average of 3.5 cases per year during this time (Heap, 2023).
A dramatic shift occurred during the 1980s to mid‐1990s with the introduction of ALS‐inhibiting (Group 2) herbicides. Products in this class, such as Pursuit and Classic, were rapidly and widely adopted by farmers. Farmers in a corn–soybean rotation could use a Group 2 herbicide in both corn and soybean for weed control and potentially develop a complete weed control program using Group 2 herbicides. Multiple companies sold Group 2 herbicides, and additional products continued to enter the marketplace while resistance issues were being detected. Herbicide resistance was first reported to Group 2 as well as the Group 1 (ACCase inhibiting) herbicides in 1982 (Heap, 2023) within five years of the introduction of these products (Shaner, 1995).
Resistance to Group 1 and Group 2 herbicides is most commonly caused by target‐site resistance (TSR). Target‐site resistance occurs when a mutation at the binding site of the target enzyme renders an herbicide ineffective because the herbicide can no longer effectively bind to the enzyme or the target enzyme is produced at such a high level that the herbicide can’t effectively bind to all sites. In contrast to the Group 5 herbicides, few fitness costs to weeds were associated with resistance to the Group 1 or 2 herbicides. The number of unique herbicide‐resistant weed cases reported globally jumped from 38 in 1980 to 180 in 1995, an average of 9.5 new cases per year during this time (Heap, 2023).
The Herbicide Resistance Action Committee (HRAC) was founded in 1989 by the agrochemical industry in response to increasing concerns around HR weeds and to support efforts in fighting HR weeds (Shaner, 1995). Herbicide resistance was also a key focus of research and various Extension efforts, which included publications, symposia, presentations, and other educational efforts, during this time (Shaner, 2014). Meanwhile, farmers were finding the need to adopt more complicated weed management programs that involved multiple modes of action and preemergence herbicides to address HR weeds.
Glyphosate‐Resistant Crops Upend the System
The weed management landscape changed dramatically with the introduction of glyphosate‐resistant (GR) crops. Farmers quickly and widely adopted the technology when GR soybeans became commercially available in 1996. Development of other GR crops followed, including GR canola and cotton in 1997, GR corn in 1998, GR alfalfa in 2005, and GR sugarbeet in 2008. Glyphosate‐based systems initially provided broad‐spectrum, effective control with little to no crop injury, and these programs were relatively easy to use and relatively inexpensive. Farmers seeding GR corn, soybean, cotton, or sugarbeet relied on glyphosate as their primary weed management tool in crop and across the cropping sequence, year after year, with little concern for carryover or drift issues. Farmers often found that they could get effective control of weeds beyond the size normally recommended for control. Reduced rates also became more common, in part to keep weed management costs as low as possible. The effectiveness of GR systems also helped contribute to a shift away from the preemergence followed by postemergence programs to total postemergence programs.
As glyphosate dominated the market, there was little incentive for chemical companies to develop other herbicide modes of action. Some argued that resistance to glyphosate would be a rare and unlikely event, and if found, resistance issues would be unlikely to develop rapidly (Shaner, 2014). On the academic front, it became even more challenging to find support for weed biology and ecology research, which should have normally helped in the development of non‐chemical weed control strategies, in part since the glyphosate system was initially so effective in controlling weeds.
Higher rates of glyphosate were often recommended, and reduced rates of residual herbicides and select tank‐mix partners were encouraged when weed control issues started to appear in fields treated with glyphosate. Debates arose about whether or not weeds were “resistant” or “tolerant” to glyphosate.
Herbicide‐Resistance Issues Continue to Expand
Herbicide resistance to glyphosate, a Group 9 herbicide, was first discovered in rigid ryegrass in Australia in 1996 and in the U.S. in 1998 (Heap, 2023). Glyphosate resistance was soon discovered in additional key weed species in the U.S., including horseweed in 2000 (Delaware), giant ragweed in 2004 (Ohio), Palmer amaranth in 2005 (Georgia and North Carolina), and waterhemp in 2005 (Missouri).
Waterhemp rose from obscurity to a major weed challenge across the upper Midwest as HR populations to Group 2 and Group 9 herbicides developed. As seen in the past with the Group 2 herbicides, weed management programs that relied heavily on the use of a single site of action, this time a Group 9 herbicide (glyphosate), resulted in the selection for weed populations that were able to survive the strategies being used.
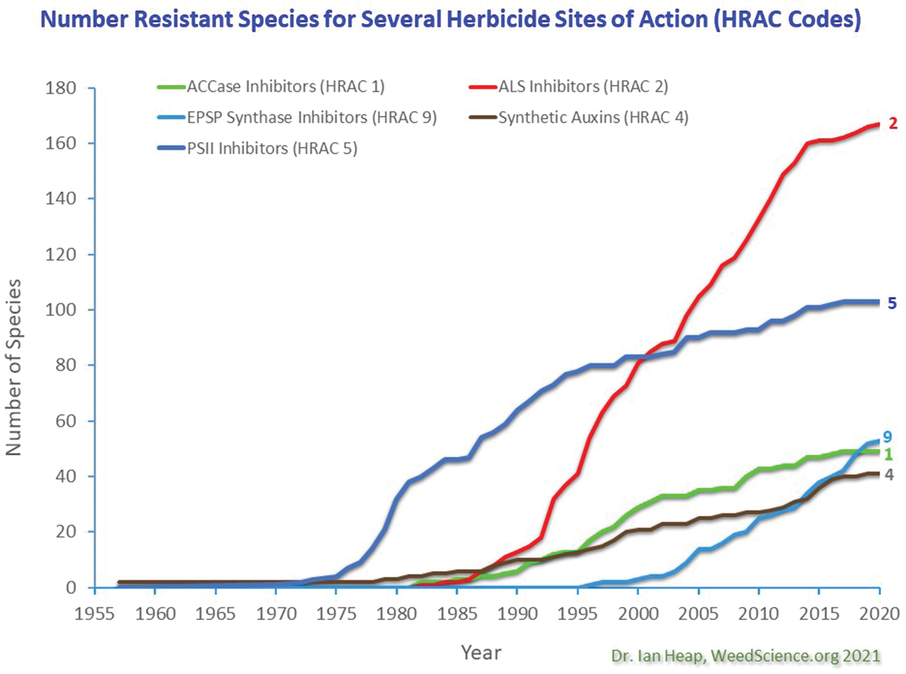
It is critical to emphasize that the increasing challenges in addressing HR weeds is not isolated to glyphosate. The number of unique HR weed cases reported globally jumped from 180 in 1995 to 518 in 2023, an average of 13.5 new cases per year during this time (Heap, 2023). As of 2022, the herbicide groups with the greatest number of reports of resistant species include the Group 2 (171 species), Group 5 (87 species), and the Group 9 herbicides (57 species) (Heap, 2023; Figure 1). Note these statistics are based on the number of cases reported: It is unknown how many unique HR weed cases remain unreported.
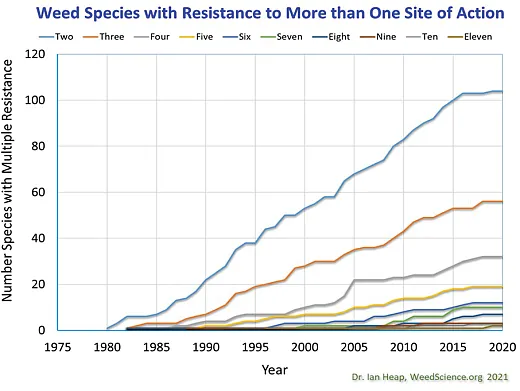
Further complicating the situation, weed populations with resistance to multiple sites of action are on the rise (Figure 2). For example, results from a survey of 85 waterhemp populations collected across Minnesota in 2021 and 2022 indicate that while 33% of the populations evaluated were resistant to one site of action, 35, 20, 8, 2, and 1% were resistant to two, three, four, five, and six sites of action, respectively, (Singh & Sarangi, 2023). In Iowa, a 2013 survey of 100 waterhemp populations found 46, 6, and 0% of populations to be resistant to three, four, and five sites of action, respectively, while a 2019 survey of 200 populations found resistance levels had increased in all three categories to 49, 14, and 4%, respectively, (Hamberg & Owen, 2022). Other states have reported populations of waterhemp resistant to at least six sites of action and populations of Palmer amaranth resistant to at least five sites of action (Heap, 2023).
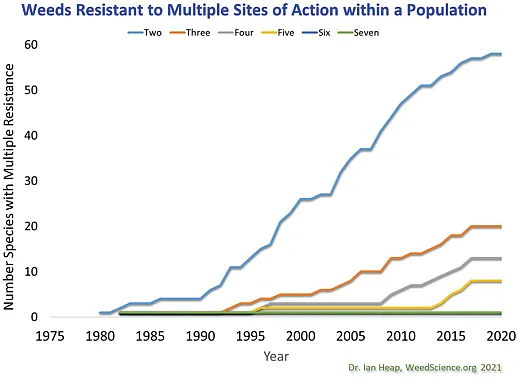
The increasing number of weed species with resistance to multiple sites of actions is an alarming trend (Figure 3), particularly as this increases the potential for populations to be found with resistance to multiple sites of action. Herbicide options become extremely limited as resistance to multiple herbicide groups stack up in a weed population.
Another complicating factor is the increasing prevalence of metabolism‐based resistance (MBR). With MBR, enzymes in the plant break down an herbicide active ingredient into metabolites that are less mobile and less toxic to the plant. The metabolites are then transported to parts of the plant, such as the cell vacuoles or the intracellular space outside the cell, where they are isolated from the target site and no longer active.
One of the greatest concerns with MBR is that it is not specific to an herbicide active ingredient (Hartzler, 2019). Once high metabolic activity is selected for, the plant may be able to break down a broad range of herbicide active ingredients, including ones the plant has never been exposed to before, as well as herbicides that have not even been discovered yet (Colquhoun, 2022). For example, Group 27 (HPPD inhibitors) resistance in waterhemp has been linked to increased activity of cytochrome P450 enzymes, something also noted in resistance to metolachlor and 2,4‐D (Hartzler, 2019). While not necessarily true in all cases, some Group 27 resistance in waterhemp may confer resistance to these other active ingredients even though they are from completely different herbicide groups. The bottom line is that MBR creates more uncertainty around the best practices to use when managing weeds with herbicides.
Additional Herbicide‐Resistant Crop Technologies Haven’t Solved the Problem
Some might conclude a solution to the growing HR weed problem is to have more HR crop technology options available to farmers. Despite the addition of the dicamba‐, 2,4‐D‐ (both Group 4 herbicides), and glufosinate‐resistant (Group 10) crop technologies, the number of HR weed species globally has continued to increase, and resistance traits are continuing to stack up in weed populations. Resistance has also been documented to these active ingredients in key weed species. Resistance in waterhemp was first documented to 2,4‐D in Nebraska in 2009 and to dicamba in 2016 in Illinois. It is important to note that the waterhemp populations involved were resistant to multiple herbicide sites of action in both cases. Resistance to dicamba has also recently been reported in kochia in North Dakota (Figure 4). To date, there have been 42 species reported worldwide with resistance to the Group 4 herbicides (Heap, 2023).

Resistance to glufosinate has been less common to date with the first case being reported in Malaysia in 2009 in goosegrass. More recently, resistance to glufosinate was reported in Palmer amaranth in 2022 in North Carolina and Arkansas.
Perception vs. Reality
The disconnect between perception of the problem and reality is evident in farmer surveys. In Minnesota, for example, farmers at Private Pesticide Applicator Recertification workshops in 2022 (n = 887) were asked if they thought they had resistance to various herbicides on land they farmed. While 67% responded that they thought they had weed resistance to glyphosate, only 16% responded they had resistance to ALS‐inhibiting (Group 2) herbicides (Stahl, Hanson, Miller, Nicolai, & Peltier, unpublished data, 2023). This contrasts with test results from 85 waterhemp populations in Minnesota that found 100% of the populations were resistant to Raptor, a Group 2 herbicide (Singh & Sarangi, 2023). Similar results were found in a survey of Iowa farmers in December 2012, regarding the presence of resistance in waterhemp populations. While waterhemp resistance to the Group 2 herbicides was documented to be widespread at the time, occurring in more than 90% of waterhemp populations, 60% of farmers and 20% of industry representatives believed it was not present in crop fields they managed.
Techno‐optimism, or the belief that technology plays a vital role in solving the world’s most pressing problems, has been identified as a barrier to overcoming HR weed problems (Dentzman et al., 2016). The belief that there will be another herbicide or HR technology to solve HR weed problems exists even though crop protection companies have released only one new mode of action since the mid‐1980s (Blois, 2022).
Can We Win the Resistance Battle?
While herbicide manufacturers and seed companies continue to seek new options and provide more diverse uses for the current herbicide portfolio, weeds continue to overcome the herbicides available. Substantial uncertainty also exists around continued use of some key tools farmers rely on for weed control, including dicamba, atrazine, and genetically modified crops.

Unfortunately, weed management and herbicide resistance are complicated, so we don’t have all the answers. What is clear is that the only way to truly stave off herbicide resistance development in our annual weed populations is to prevent seed production, a high bar for any farmer to achieve. University of Minnesota research demonstrated that the giant ragweed seed bank could be depleted by 97% in two years while University of Illinois research found the waterhemp seed bank could be depleted by more than 99% in four years (Goplen et al., 2017; Stahl et al., 2017). These results show how moving our focus to preventing seed production or additions to the soil seed bank can produce real results that farmers can realize in a relatively short time frame, perhaps in only two to four years for some species. Farmers must focus on best using the herbicide technologies available while also expanding their weed management strategies to include non‐chemical tactics that complement herbicides with the focus on preventing seed production to stop the spread of HR weeds.
The first step in better weed management is to prevent the introduction and spread of weeds that may contain HR traits. While the concept seems simple, short‐ and long‐distance movement of feed, equipment, and soil means the potential for new introductions always exists. Careful monitoring and management in areas at highest risk, like feed storage areas and field edges and entrances, can help prevent new weeds from spreading.

The next step is focusing on getting improved value from the herbicides that are currently available. A comprehensive review of Illinois waterhemp populations found that using multiple sites of action in mixtures reduced the selection for glyphosate resistance while rotating chemistries and using fewer herbicide sites of action per year increased the presence of glyphosate resistance (Evans et al., 2016). While this works well for populations with TSR, Aaron Hager, weed scientist with the University of Illinois noted in a recent article how little we know about managing MBR in weed populations (Hager, 2023).
Farmers must choose the most effective product mixtures against the weed spectrum in a given field and apply them at full‐labeled rates. The best‐laid plans can fall apart on the follow through, so farmers need to set up products to perform their best, including using the correct nozzles, carrier volume, adjuvants, and timing. Given that the priority for effective resistance management requires that no weed seed be produced, other tactics aside from herbicides are instrumental to success. Herbicides cannot save us from a problem that herbicides have caused. Period.
The final step is to introduce diversity into the system, which farmers can accomplish via numerous cultural and mechanical methods (Table 1). Many farmers are likely to choose complementary strategies that will work in their current, herbicide‐based system. Cultural strategies may include cover crops, narrow‐row soybean, and delayed planting (e.g., for giant ragweed). Mechanical methods to remove weeds and their seed include tillage, hand rouging, weed electrification, and harvest weed seed control, to name a few. These methods can all be effective to further reduce the number of weed seed returning to the soil.
Cultural | Mechanical |
|---|---|
| Prevention (e.g., manage weeds at field borders, avoid harvesting weedy areas within the field) | Tillage (e.g., interrow cultivation, rotary hoe) |
| Cover crops | Hand rouging |
| Crop rotation | Physical weed control devices (e.g. mowers, flame, electrical discharge, pullers, etc.) |
| Crop competitiveness (e.g., narrow rows) | Harvest weed seed control (e.g. seed destruction devices, chaff lining and tramlining) |
Recently, numerous universities have researched diversification techniques that can be complementary to herbicides like using narrow rows and cereal rye cover crops for weed suppression in soybean. One recent project in Iowa found that rotations with a highly effective corn herbicide program, a cereal rye cover crop, and narrow‐row soybean resulted in 87% less waterhemp emergence than those with a marginal corn herbicide program, no cover crop, and 30‐inch rows in soybean (Yadav et al., 2023; Figure 5). Additionally, significant research is being conducted on the incorporation of mechanical strategies to either physically remove or kill weeds or to reduce additions to the seed bank at the end of the growing season. These additional weed management techniques show significant promise, particularly in the potential to use them together to manage weeds.

The most robust strategy of diversification will include multiple strategies with crop rotation as the keystone. A crop rotation over multiple years that contains crops growing during different seasons allows farmers to exploit weaknesses in weed biology without the need for greater herbicide reliance. Research has shown the benefit of diversification beyond a typical corn–soybean rotation on reducing reliance on herbicides (Davis et al., 2012) and reducing weed density in crop fields (Weisberger et al., 2019).
The challenge with implementing these non‐chemical strategies is often related to cost, ease of use, and feasibility in a system we have simplified to allow for maximum efficiency. To many, even the simple step of improving control by using a more diversified herbicide program will mean higher costs to achieve only marginally better weed control. How do we then emphasize the importance of a long‐term view and the need for diversification? Integrating multiple strategies with the goal of preventing seed additions to the soil seed bank is the best strategy to maintain the ability to continue a cropping system into the foreseeable future.
Research must continue in order to better understand how to stave off herbicide resistance and most effectively use the tools available to diversify systems. Technology has come a long way, but a collaborative effort among herbicide manufacturers, seed producers, engineers, weed scientists, farmers, crop advisers, and others to maximize the efficiency of using diversified tools will be critical for implementation and long‐term success.
Conclusions
While we know herbicide resistance is an inevitable fate in a system that relies heavily on herbicides, we can work together to focus on long‐term strategies to delay its development and spread. Managing for a single year is a key reason we have gotten into the position we are in. Farmers rely heavily on their advisers to provide sound agronomic recommendations, and we, as advisers, need to take that role seriously to broach the subject of long‐term weed management beyond individual‐year herbicide recommendations. We have a collective responsibility to preserve the value of herbicides by ensuring successful diversification of weed management programs, working as partners together.
Part of ensuring successful diversification is educating ourselves on strategies that can be successful in our geographies. Herbicide resistance in weeds is not going away, and it cannot be prevented as long as herbicides are a cornerstone of our weed management programs. The advice crop advisers give to their customers, the information provided by academia, the strategies promoted by industry, and ultimately the practices employed by farmers can all make a difference in helping determine the longevity of the tools we have available today as well as what options we will have available in the future.
Call to Action
Don’t let weeds go to seed. Scout, properly identify, and map your weeds. Use non‐chemical strategies for weed control (see Table 1). Use weaknesses in the biology of weed species to your advantage (e.g. short lifetime in the seed bank, early emergence). Use crop canopy/competition to improve weed control. Follow the herbicide label to maximize efficacy of the product. When using herbicides, use multiple sites of action within a year. Apply effective preemergence herbicides at full rates. Regularly evaluate and adjust weed management strategies, working with a long‐term/multi‐year plan. Consider getting the Resistance Management Specialty (RMS) certification (certifiedcropadviser.org/rms). This additional specialty certification demonstrates a CCA’s proficiency in working with the RM concept and builds into it a holistic management model.
Resources
Take Action Herbicide Classification Chart: https://iwilltakeaction.com/resources/herbicide‐classification‐chart
University of Minnesota Extension Herbicide Resistance Management Website: https://extension.umn.edu/weed‐management/herbicide‐resistance‐management
Weed Science Society of America Herbicide Resistance Website: https://wssa.net/wssa/weed/resistance
Iowa State University Integrated Crop Management Page: https://crops.extension.iastate.edu
SARE publication Manage Weeds on Your Farm. A Guide to Ecological Strategies: https://www.sare.org/wp‐content/uploads/Manage‐Weeds‐on‐Your‐Farm.pdf
References
Blois, M. (2022, June 17). Following several fallow decades, herbicide companies are searching for new modes of action. Chemical & Engineering News. https://cen.acs.org/environment/pesticides/crop‐protection‐herbicide‐mode‐action‐glyphosate/100/i22
Colquhoun, J. (2022). Is metabolic herbicide resistance the straw that will break weed management’s back? http://ipcm.wisc.edu/blog/2022/09/metabolic‐herbicide‐resistance
Davis, A.S., Hill, J.D., Chase, C.A., Johanns, A.M., & Liebman, M. (2012). Increasing cropping system diversity balances productivity, profitability and environmental health. PLoS ONE 7(10), e47149. https://doi.org/10.1371/journal.pone.0047149
Dentzman, K., Gunderson, R., & Jussaume, R. (2016). Techno‐optimism as a barrier to overcoming herbicide resistance: Comparing farmer perceptions of the future potential of herbicides. Journal of Rural Studies, 48, 22–32.
Evans, J.A., Tranel, P.J., Hager, A.G., Schutte, B., Wu, C., Chatham, L.A., & Davis, A.S. (2016). Managing the evolution of herbicide resistance. Pest Management Science, 72, 74–80.
Goplen J.J., Sheaffer, C.C., Becker, R.L., Coulter, J.A., Breitenbach, F.R., Behnken, L.M., Johnson, G.A., & Gunsolus, J.L. (2017). Seedbank depletion and emergence patterns of giant ragweed (Ambrosia trifida) in Minnesota cropping systems. Weed Science, 65, 52–60.
Gunsolus, J. (2021). Herbicide‐resistant weeds. University of Minnesota Extension. https://extension.umn.edu/herbicide‐resistance‐management/herbicide‐resistant‐weeds
Hager, A. (2023). Recommendations to manage herbicide‐resistant weeds: it’s not as easy as some believe. Department of Crop Sciences, University of Illinois. https://farmdoc.illinois.edu/field‐crop‐production/weeds/recommendations‐to‐manage‐herbicide‐resistant‐weeds‐its‐not‐as‐easy‐as‐some‐believe.html
Hamberg, R., & Owen, M.D.K. (2022). Frequency and distribution of herbicide‐resistant common waterhemp populations in Iowa. Iowa State University Integrated Crop Management Field Notes (FIELD 23–118). https://www.aep.iastate.edu/fieldnotes/notes/118.pdf
Hartzler, B. (2019). Metabolism‐based resistance—Why the concern? Iowa State University Extension & Outreach. http://crops.extension.iastate.edu/blog/bob‐hartzler/metabolism‐based‐resistance‐why‐concern
Heap, I. (2023). The International Herbicide‐Resistant Weed Database. http://www.weedscience.org/
Shaner, D.L. (1995). Herbicide resistance: Where are we? How did we get here? Where are we going? Weed Technology, 4, 850–856.
Shaner, D.L. (2014). Lessons learned from the history of herbicide resistance. Weed Science, 62, 427–431.
Singh, N., & Sarangi, D. (2023). Weed management update. Research update for ag professionals. University of Minnesota Extension.
Stahl, L., Goplen, J., & Behnken, L., (2017, April 26). It’s not all about herbicides: Three key tactics for managing weeds. Minnesota Crop News. https://blog‐crop‐news.extension.umn.edu/2017/04/its‐not‐all‐about‐herbicides‐three‐key.html
Weisberger, D., Nichols, V., & Liebman, M. (2019). Does diversifying crop rotations suppress weeds? A meta‐analysis. PLoS ONE 14(7), e0219847. https://doi.org/10.1371/journal.pone.0219847
Yadav R., Jha, P., Hartzler, R., & Liebman, M. (2023). Multi‐tactic strategies to manage herbicide‐resistant waterhemp (Amaranthus tuberculatus) in corn–soybean rotations of the U.S. Midwest. Weed Science, 71, 141–149.
Self-Study CEU Quiz
Earn 1.5 CEUs in Integrated Pest Management by taking the quiz. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
- Herbicide resistance was first reported in 1957 to
- field bindweed and Canada thistle.
- common lambsquarters and leafy spurge.
- wild carrot and climbing dayflower.
- velvetleaf and giant ragweed.
- The term “herbicide mode of action” can be defined as
- the specific biochemical site affected by the herbicide.
- the biological process affected by the herbicide.
- the breaking down of enzymes into less mobile units.
- the speed of herbicides into plant cell vacuoles.
- Target-site resistance occurs when a __________ occurs at the binding site or the target enzyme renders an herbicide ineffective.
- blockage
- conjugation
- mutation
- enzyme
- Metabolism-based herbicide resistance can be defined as the breakdown of herbicide active ingredients into metabolites that are
- less mobile and less toxic to the plant.
- more mobile and less toxic to the plant.
- less mobile and more toxic to the plant.
- more mobile and more toxic to the plant.
- How many weed species have been reported resistant to Group 4 herbicides worldwide as of 2023?
- 15.
- 26.
- 42.
- 51.
- Research has determined that the giant ragweed weed seed bank can be depleted via integrated weed management to what level after two years?
- 60%.
- 75%.
- 83%.
- 97%.
- A review of Illinois waterhemp populations by the University of Illinois indicated that using multiple sites of action in a year reduced the selection for glyphosate resistance compared with rotating chemistries across years. Herbicide resistance continues to be on the rise, however, likely due in part to this kind of resistance:
- Target-site resistance.
- Metabolic resistance.
- Conjugation.
- Limited translocation.
- Which weed management strategy is NOT recommended as a successful cultural practice?
- Cover crops.
- Crop rotation.
- Tillage.
- Crop competitiveness.
- A recent Iowa research project implementing a diversification approach for weed control resulted in an 87% reduction in waterhemp. Which of the following strategies was NOT part of the approach?
- A highly-effective corn herbicide program.
- A cereal rye cover crop.
- Narrow-row soybeans.
- 30-inch row soybeans.
- According to research in North Dakota, kochia has developed resistance to dicamba applications at a higher-than-normal use rate of
- 0.05 lb active ingredient.
- 1.125 lb active ingredient.
- 0.25 lb active ingredient.
- 0.5 lb active ingredient.
- Which of the following is a mechanical form of weed control?
- Cover crops.
- Scouting weeds.
- Harvest weed seed control.
- Avoiding harvest of weedy patches.
- Which herbicide group has had the second-most number of reports of resistant species worldwide as of 2023?
- Group 1.
- Group 2.
- Group 5.
- Group 9.
- Weeds can be resistant to herbicides that have not yet been put on the market.
- True.
- False.
- What percentage of the waterhemp populations tested in a 2019 Iowa State University survey were found to have resistance to five sites of action?
- 4%.
- 1%.
- 6%.
- 2%.
- Test results from a Minnesota evaluation of waterhemp populations found that what percent of the populations had resistance to a Group 2 herbicide?
- 67%.
- 16%.
- 90%.
- 100%.
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.









