Phosphite use in a declining fungicide market


Phosphite has been a controversial topic for years. Is it fertilizer, a biostimulant, or a fungicide? Looking carefully, one can conclude that phosphite serves all three functions. In this article, we’ll focus on the use of phosphite as a fungicide. As with everything we do with chemicals and nutrition, we need to be aware of possible negative effects, and we also need to determine how we are using the phosphite materials and the results we are seeking. Earn 0.5 CEUs in Integrated Pest Management by reading the article and taking the quiz.
Phosphite has been a controversial topic for years. Its use and benefits are argued in hundreds of research papers across the world’s scientific communities. Is it fertilizer, a biostimulant, or a fungicide? These questions are discussed in multiple university research results. I believe that if we look carefully, we can conclude phosphite serves all three functions. As with everything we do with chemicals and nutrition, we need to be aware of possible negative effects. We also need to determine how we are using the phosphite materials and the results we are seeking.
Phosphite (PO33–), a reduced form of phosphate (PO43–), is widely marketed as either a fungicide or fertilizer or sometimes as a biostimulants. Because crop consultants, growers, and distributors see the product marketed in all three ways, it can cause confusion. Each designated use will most likely be tied to the phenology (growth stage) of each crop treated. Timing is critical to determine which effect we are seeking. Previous articles have addressed phosphite in a plant nutrition role. Additional articles speak of phosphite in terms of a biostimulant.
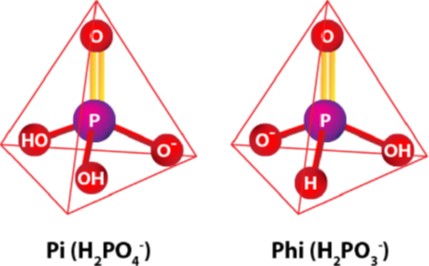
In this article, we broach the controversial topic of phosphites as a fungicide. Phosphite’s history since the 1970s has given rise to many fungicide brand options for agriculture use. USEPA-registered labels exhibit several active ingredient versions of the phosphonate category, which are all derived from various manufacturing processes. Phosphonate is a general collective term used for the salts and esters of phosphites. This will also include phosphorous acid, phosphoric acid, and phosphonic acid.
Mode of action against pathogens
Phosphite salts can be used as a biodegradable fungicide to protect plants against Phytophthora dieback. It is usually applied as potassium phosphite, derived from phosphorus acid neutralized with potassium hydroxide. Calcium, aluminum, ammonium, and magnesium phosphite may also be used. The phosphonate fungicide group disrupts the Phytophthora fungus through interference of phosphate metabolism. Polyphosphate and pyrophosphate accumulate in the cells, which divert ATP from critical metabolic pathways. The result is decreased growth of the Phytophthora fungus. Oomycetes pathogens, in addition to Phytophthora, may be controlled or inhibited directly or indirectly with applications of phosphites.
A known indirect method of fungal control of how phosphite works is by boosting the plant's own natural defenses and thereby allowing susceptible plants to survive within sites that are infested with Phytophthora dieback. It is important to note that there is no treatment that will eradicate Phytophthora dieback, including phosphite.

Phosphite can enhance plant health directly through control of selected fungi on cultivated or native plants. In general, phosphite acts as a priming agent of several plant defense responses. The plant’s disease defense response is referred to as systemic acquired resistance (SAR). Cell-to-cell signaling triggers a dramatic increase in gene expression. A 2021 study showed phosphite applied to potatoes upregulated 2,856 different genes. Excellent reviews on the use of phosphite to control or reduce the severity of selected plant diseases have been published. Phosphite-based fungicides often are labeled as fertilizers because of significantly less complex and costly regulatory approval processes required for fertilizers compared with fungicides.
Use of phosphite as a fungicide is primarily targeted to control of oomycete pathogens like those of Phytophthora. It has been shown to control many others. In studies, growth of a phosphite-sensitive strain was inhibited regardless of phosphorus supply, whereas resistant strains were inhibited by phosphite only under low phosphate levels. Phosphite is effective in controlling root and crown rot caused by Phytophthora, and researchers reported a linear reduction in the severity of downy mildew and a significant improvement in soybean with an increase. Significant phosphite control of phytophthora disease in citrus may be achieved through both soil and foliar applications. Soil-applied phosphite was more effective in controlling citrus root rot. In addition, some nematode control has been documented.
Wide range of control
The concentration of phosphite at the infection site is key. At lower levels of concentration, antifungal metabolism can be triggered, and at higher concentration, phosphite can inhibit fungal growth. Ink disease in walnuts was significantly reduced through some treatment methods. Foliar-applied phosphite has been successful in controlling pecan scab. Phosphite has also shown it can be effective in crown rot on peppers. Fungicides containing phosphite can suppress foliar and soil-borne diseases. With foliar diseases, repeated applications are frequently needed as phosphite should be present at the time an infection occurs. In contrast to non-systemic fungicides (e.g., mancozeb) labeled for oomycetes, phosphite is readily translocated throughout the plant, which is especially advantageous for disease control in potato tubers and other underground plant tissue in potatoes. Phosphite has been foliar-applied during the growing season or sprayed on potatoes post-harvest both with excellent fungal disease control.
Interestingly, phosphite has been shown effective against summer decline in creeping bentgrass by Pythium. Root and shoot growth improvements have been observed in turf management along with good control on dollar spot. Additionally, positive control of Pythium in bluegrass was observed. Pythium blight suppression due to phosphite treatments on perennial ryegrass and creeping bentgrass and anthracnose basal rot on an annual bluegrass were reported.
As seen below, the benefits of phosphite in the control of numerous diseases have been researched for years (Table 1). The number of research reports is too numerous to go into in a short review. We encourage CCAs and PCAs to research this effective tool on their own and find the fit in the crops they manage. We need all the tools at our disposal, and phosphites are proven by research to be effective. The phosphonate chemistry is designated as a Group 33 fungicide. Consider using this active ingredient with your fungicide program to help prevent or delay disease resistance.
| Plant disease | Causal agent |
|---|---|
| Apple mouldy core | Alternaria alternate |
| Avacado dieback | Phytophthora cinnamomi |
| Banksia dieback | Phytophthora cinnamomi |
| Bentgrass summer decline | Pythium spp. |
| Cabbage clubroot | Plasmodiophora brassicae |
| Chestnut ink disease | Phytophthora cambivora |
| Cucumber damping-off | Pythium ultimum |
| Grape downy mildew | Plasmopara viticola |
| Lupin dieback | Phytophthora cinnamomi |
| Maize downy mildew | Peronosclerospora sorghi |
| Orange brown rot | Phytophthora citrophthora |
| Papaya fruit rot | Phytophthora palmivora |
| Pecan scab | Fusicladium effusum |
| Pepper crown and root rot | Phytophthora capsici |
| Potato late blight | Phytophthora infestans |
| Potato pink rot | Phytophthora erythroseptica |
| Potato bacterial soft rot | Erwinia carotovora |
| Soybean downey mildew | Peronospora manshurica |
| Strawberry leather rot | Phytophthora cactorum |
| Tangelo brown spot | Alternaria alternata |
| Tobacco black shank | Phytophthora nicotiana |
Phosphite performance will vary from suppression to control of certain fungal pathogens. Keep this in mind if you use them without a tank mix partner. Since all phosphites are not created equal, we advise caution to use them correctly. Without special stabilization through the manufacturing process, they do not mix well with metal micronutrients or metal-containing fungicides and pesticides. There is currently technology that allows you to mix phosphites safely and effectively. To learn more about a special patented technology that can stabilize your phosphite and allow safe tank-mixing and complete efficacy of applied products, visit this site or call Verdesian Life Science at 559-203-0976.
Adams, F., & Conrad, J. (1953). Transition of phosphite to phosphate in soils. Soil Science, 75, 361–371.
Benitez-Nelson, C. (2000). The biogeochemical cycling of phosphorus in marine systems. Earth-Science Reviews, 51, 109–135.
Bezuidenhout, J., Darvas, J., & Kotze, J. (1987). The dynamics and distribution of phosphite in avocado trees treated with phosetyl-Al. South African Avocado Growers’ Association Yearbook, 10, 101–103.
Cade-Menun, B., Berch, S., Preston, C., & Lavkulich, L. (2000). Phosphorus forms and related soil chemistry of Podzolic soils on northern Vancouver Island: A comparison of two forest types. Canadian Journal of Forest Research, 30, 1714–1725.
Casida, L. (1960). Microbial oxidation and utilization of orthophosphite during growth. Journal of Bacteriology, 80, 237–241.
Chemjet. (n.d.). Phosphite (phosphonate) fungicide treatment. https://www.chemjet.co.uk/Wikipedia-entry-on-Phosphite-fungicide-treatment--pg36.aspx
Condron, L. M., & Newman, S. (2011). Revisiting the fundamentals of phosphorous fractionation of sediments and soil. Journal of Soils and Sediments, 11, 830–840.
Costas, A., White, A., & Metcalf, W. (2001). Purification and characterization of a novel phosphorus-oxidizing enzyme from Pseudomonas stutzeri WM88. Journal of Biological Chemistry, 276, 17429–17436.
Figueroa, I., & Coates, J. (2017). Microbial phosphite oxidation and its potential role in the global phosphorus and carbon cycles. Advances in Applied Microbiology, 98, 93–117.
Foster, T., Winans, L., & Helms, S. (1978). Anaerobic utilization of phosphite and hypophosphite by Bacillus sp. Applied and Environmental Microbiology, 35, 937–944.
GCSAA. (2018). Study 1: phosphite fungicides and fertilizers and contact fungicides. GCM. https://www.gcmonline.com/docs/librariesprovider2/research-charts/2018/phosphite-fungicides-and-fertilizers.pdf
Glindemann, D., Bergmann, A., Stottmeister, U., & Gassmann, G. (1996). Phosphine in the lower terrestrial troposphere. Naturwissenschaften, 83, 131–133.
Golf Ventures. (2021). Phosphites fungicide products for golf courses. Golf Ventures. https://www.golfventures.com/store/golf-course-maintenance-supplies/chemicals/fungicides/phosphites/
The Grass Factor. (2021). Phosphites for lawns–fertilizer or fungicide? YouTube. https://www.youtube.com/watch?v=XszdgYC5CuQ
Guest, D., & Grant, B. (1991). The complex action of phosphonates as antifungal agents. Biological Reviews, 66, 159–187.
Hanrahan, G., Salmassi, T., Khachikian, C., & Foster, K. (2005). Reduced inorganic phosphorus in the natural environment: Significance, speciation and determination. Talanta, 66, 435–444.
Havlin, J. L., & Schlegel, A. J. (2021). Review of phosphite as a plant nutrient and fungicide. Soil Systems, 5(3), 52. https://doi.org/10.3390/soilsystems5030052
Hedley, M., Stewart, J., & Chauhan, B. (1982). Changes in the inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubation. Soil Science Society of America Journal, 46, 970–976.
Howard, K., Dell, B., & Hardy, G. (2001). Phosphite and mycorrhizal formation in seedlings of three Australian Myrtaceae. Australian Journal of Botany, 48, 725–729.
Huang, L., Jia, X., Zhang, G., & Shao, M. (2017). Soil organic phosphorus transformation during ecosystem development: A review. Plant and Soil, 417, 17–42.
Imazu, K., Tanaka, S., Kuroda, A., Anbe, Y., Kato, J., & Ohtake, H. (1998). Enhanced utilization of phosphonate and phosphite by Klebsiella aerogenes. Applied and Environmental Microbiology, 64, 3754–3758.
Jackman, R., Lambert, J., & Rothbaum, H. (1970). Red phosphorus as a fertiliser for grass-clover pasture. New Zealand Journal of Agricultural Research, 13, 232–241.
Lindsay, W. L. (1979). Chemical Equilibria in Soils. John Wiley & Sons, New York, NY.
Loera-Quezada, M., Leyva-Gonzalez, M., Lopez-Arredondo, D., & Herrara-Estrella, L. (2015). Phosphite cannot be used as a phosphorus source but is non-toxic for microalgae. Plant Science, 231, 124–130.
Lovatt, C., & Mikkelsen, R. (2006). Phosphite fertilizers: What are they? Can you use them? What can they do? Better Crops, 90, 11–13.
MacIntire, W., Winterberg, S., Hardin, L., Sterges, A., & Clements, L. (1950). Fertilizer evaluation of certain phosphorus, phosphorous, and phosphoric materials by means of pot cultures. Agronomy Journal, 42, 543–549.
Malacinski, G., & Konetzka, W. (1966). Bacterial oxidation of orthophosphite. Journal of Bacteriology, 91, 578–582.
McDonald, A., Grant, B., & Plaxton, W. (2001). Phosphite (phosphorous acid): Its relevance in the environment and agriculture and influence on plant phosphate starvation response. Journal of Plant Nutrition, 24, 1505–1519.
Metcalf, W., & Wolfe, R. (1998). Molecular genetic analysis of phosphite and hypophosphite oxidation by Pseudomonas stutzeri WM88. Journal of Bacteriology, 180, 5547–5558.
Metcalf, W., & Wanner, B. (1991). Involvement of the Escherichia coli phn (psiD) gene cluster in assimilation of phosphorus in the form of phosphonates, phosphite, Pi esters, and Pi. Journal of Bacteriology, 173, 587–600.
Morton, S., & Edwards, D. (2005). Reduced phosphorus compounds in the environment. Critical Reviews in Environmental Science and Technology, 35, 333–364.
Morton, S., Glindemann, D., & Edwards, M. (2003). Phosphates, phosphites, and phosphides in environmental samples. Environmental Science and Technology, 37, 1169–1174.
Morton, S., Glindemann, D., Wang, X., Niu, X., & Edwards, M. (2005). Analysis of reduced phosphorus in samples of environmental interest. Environmental Science and Technology, 39, 4369–4376.
Ohtake, H., Wu, H., Imazu, K., Anbe, Y., Kato, J., & Kuroda, A. (1996). Bacterial phosphonate degradation, phosphite oxidation and polyphosphate accumulation. Resources, Conservation and Recycling, 18, 125–134.
Pao, S., Paulsen, I., & Saier, M. (1998). Major facilitator superfamily. Microbiology and Molecular Biology Reviews, 62, 1–34.
Pasek, M. (2008). Rethinking early Earth phosphorus geochemistry. Proceedings of the National Academy of Sciences of the United States of America, 105, 853–858.
Pasek, M., Harnmeijer, J., Buick, R., Maheen, G., & Atlas, Z. (2013). Evidence for reactive reduced phosphorus species in the early Archean ocean. Proceedings of the National Academy of Sciences of the United States of America, 110, 10089–10094.
Pasek, M., Sampson, J., & Atlas, Z. (2014). Redox chemistry in the phosphorus biogeochemical cycle. Proceedings of the National Academy of Sciences of the United States of America, 111, 15468–15473.
Penn State Extension. (2023). Understanding the phosphonate products. https://extension.psu.edu/understanding-the-phosphonate-products
Rawat, P., Das, S., Shankhdhar, D., & Shankhdhar, S. (2020). Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. Journal of Soil Science and Plant Nutrition, 21, 49–68.
Rodríguez, H., & Fraga, R. (1999). Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances, 17, 319–339.
Rafi, M., Krishnaveni, M., & Charyulu, P. (2019). Phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture. In S. Buddolla (Ed.), Recent Developments in Applied Microbiology and Biochemistry (pp. 223–235). Elsevier, London, UK.
Schink, B., & Friedrich, M. (2000). Bacterial metabolism: Phosphite oxidation by sulphate reduction. Nature, 406, 37.
Schink, B., Thiemann, V., Laue, H., & Friedrich, M. (2002). Desulfotignum phosphitoxidans sp. nov., a new marine sulfate reducer that oxidizes phosphite to phosphate. Archives of Microbiology, 177, 381–391.
Simeonova, D., Wilson, M., Metcalf, W., & Schink, B. (2010). Identification and heterologous expression of genes involved in anaerobic dissimilatory phosphite oxidation by Desulfotignum phosphitoxidans. Journal of Bacteriology, 192, 5237–5244.
Thao, H. T. B., & Yamakawa, T. (2009). Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Science and Plant Nutrition, 55(2), 228–234. https://doi.org/10.1111/j.1747-0765.2009.00365.x
Vilela, A. E., de Resende, M. L. V., de Medeiros, F. C. L., de Brito Pereira, M. H., Santiago, W. D., de Azevedo Santos, L., dos Santos Botelho, D. M., & Ramalho, T. C. (2022). Association phosphite x fungicide: Protection against powdery mildew in soybean plants, translocation and computer simulation. Journal of Plant Pathology, 104, 787–793. https://doi.org/10.1007/s42161-022-01086-2
Wells, L. (2020). Clarification on phosphite materials. University of Georgia Extension. https://site.extension.uga.edu/pecan/2020/03/clarification-on-phosphite-materials/
Self-study CEU quiz
Earn 0.5 CEUs in Integrated Pest Management by taking the quiz for the article. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
The fungicidal properties of the phosphonate chemistry class
a. allow it to be used alone in a fungicide program.
b. make it Ineffective against oomycete pathogens.
c. allow it to be used with a tank mix partner.
d. prevent it from being translocated throughout the plant.
e. Both a and c.
f. Both b and d.
2. Phosphites control or suppress fungal pathogens by
a. direct and indirect methods.
b. altering soil pH.
c. acting as an herbicide.
d. None of the above.
Which class of pathogens do phosphites primarily target?
a. Revtraviricetes (mosaic virus).
b. Oomycetes (water molds).
c. Ustilaginomycetes (smuts).
d. Helminths.
Mitigating disease organisms with phosphites can occur by interference of phosphate metabolism and upregulation of multiple genes.
a. True.
b. False.
Phosphite is recognized as a
a. fertilizer.
b. biostimulant.
c. fungicide.
d. All the above.
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.