Assessing Soil Potassium: Basic Principles and New Developments

Potassium (K) is an essential element for crop growth, playing a key role in photosynthesis, protein synthesis, and many other essential functions in the plant. In 2020, 44% of the soils sampled across North America tested below critical in K (meaning applying K fertilizer would result in a crop response). Assessing soils for nutrient availability is an important principle of 4R Nutrient Stewardship. The goal of this article is to provide information on the behavior of K in soils and how it influences the assessment of crop K needs. Earn 1 CEU in Nutrient Management by reading this article and taking the quiz at https://web.sciencesocieties.org/Learning‐Center/Courses.
Potassium (K) is an essential element for crop growth and is considered a macronutrient because plants take up large amounts of K throughout their growth. Potassium plays a key role in photosynthesis, protein synthesis, and many other essential functions in the plant. For perennial crops, like alfalfa, K is especially important for promoting stand survival through winter. In 2020, 44% of the soils sampled across North America tested below critical in K (meaning applying K fertilizer would result in a crop response). Assessing soils for nutrient availability is an important principle of 4R Nutrient Stewardship. The goal of this article is to provide information on the behavior of K in soils and how it influences the assessment of crop K needs.
Soil Pools of K
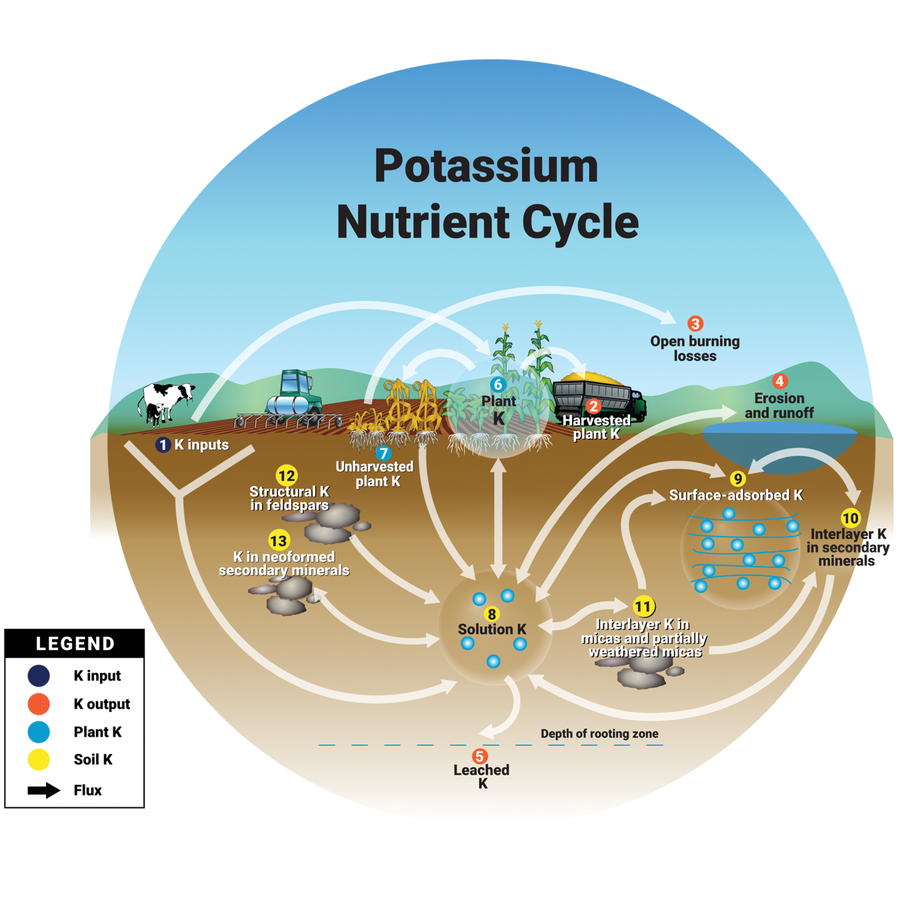
Soils frequently contain more than 20,000 ppm (parts per million) of K, but only a fraction of that K is available for crop uptake. There are three major pools of K in soils. They correspond to the numbered pools indicated in Figure 1 and include:
- 12 and 13—mineral K (unavailable),
- 10 and 11—secondary minerals and compound K (slowly available), and
- 8 and 9—exchangeable K (soil solution and surface‐sorbed) (readily available).
The mineral K fraction makes up roughly 90 to 98% of the total potassium in soils. This form of potassium is locked up in the minerals in soils that are resistant to weathering, or breakdown. The K in the mineral fraction slowly becomes available as the minerals weather, but this process can take hundreds of years, and the amount of available K added to the soil solution is small.
Secondary minerals contain K that is considered slowly available. This K is often referred to as “fixed K” and is held in the interlayers of clay minerals. As soils become wet or dry, the layers of clay expand or shrink, allowing K to escape or become trapped, which is known as “K fixation.”
The final pool is the exchangeable K, which is sometimes classified as two pools: exchangeable K and soil solution K though all K in this group is available for plant uptake. The exchangeable K is held on the cation exchange complex. The cation exchange complex is the negatively charged sites on clays and organic matter that hold the positively charged K cation. The number of negatively charged sites determines a soil’s cation exchange capacity (CEC). The exchangeable K is in constant equilibrium with the soil solution K. The soil solution is the water phase in soils and contains many nutrients, including K, that are readily available to the plant through diffusion. As plants take up K and remove it from the soil solution, the K from the CEC quickly replenishes it to restore equilibrium.
While the amount of available K for crop uptake may seem straight forward, the slowly available K or fixed K from clay minerals can complicate things.
Clay Mineralogy Basics
Each soil consists of sand, silt, and clay. But within the clay fraction, there are dozens of types of clay minerals. The type of clay in a soil largely determines the CEC of that soil. Clay minerals are unique in that they are layered and stack on top of one another. Under the microscope, clay minerals look like pages of a book (Figure 2). The space between layers (or pages in the book following the analogy) and chemical makeup of the various clay minerals cause potassium ions (K+) to behave differently between clay types.

While there are many clay types out there, two broad classes that have been studied for their influence on K are smectite and illite (also known as mica). Illite minerals have a moderate CEC and an interlayer space that traps (or fixes) K. Smectites, on the other hand, have a very high CEC and an interlayer space that shrinks and swells based on the water content of the soil. The shrink–swell behavior of smectites can trap or release K, depending on the soil moisture level.
The relative amounts of illite and smectite in a particular soil can influence the interpretation of the soil test for K. Research in North Dakota (Breker et al., 2019) concluded that for soils with a ratio of smectite to illite less than 3.5, the critical soil test level was 130 ppm compared with 200 ppm for those with higher ratios. Research in Illinois and Indiana showed that soil wetness could interact with clay type since soil conditions leading to chemical reduction of iron (generally, wet, flooded, and anaerobic conditions) were observed to increase K fixation in soils with montmorillonite (a kind of smectite) (Stucki, 1996).
Soil Test K, Clay Minerals, and Soil Water
The differences between clay types are suspected to contribute to differences in K reported on soil tests. In the United States, dried soil samples are the standard for routine soil tests. However, research from Iowa State University has found that using moist soil samples may lead to more accurate soil test K (Oltmans & Mallarino, 2015).
Testing non‐dried samples (that is, keeping the soil water content constant from sampling) is not new, and it has been known for decades that drying soils might affect the extraction of certain nutrients, especially K. In soils with smectite clays, drying the soil drives water out of the interlayers and traps K between the clay layers. However, in illite clay, the drying causes the clay layers to curl, releasing the “fixed” K. Because of the clay behavior, scientists know that drying the soil can impact the amount of K extracted.
However, testing non‐dried soil samples for K is not common. Many soil labs are not equipped to store, handle, or analyze field‐moist samples. Since moist samples cannot as easily be ground and mixed, the analytical procedures require larger subsamples for extraction and thus require more reagents, increasing costs. Furthermore, K recommendations are built from datasets and research using dried soil samples. Using moist soils tested for K with recommendations for dried soils will lead to misapplications of K. Each nutrient recommendation is built for specific soil test methods and extractants. Research in Iowa showed that critical soil K levels were lower for soils tested at field moisture than for those that had been dried (Barbagelata & Mallarino, 2013).
Moist K soil tests tell us that clay mineralogy does influence how K is available in the soil. The type of clay in your soil may be impacting K availability. However, until recommendations are built around K for moist soils or clay type, follow guidance from your soil test lab or state extension service for soil sample handling and interpretation based on soil clay type.
Soil Sampling for K Levels
Potassium availability is tangled with more than the type of clay minerals in soils. Timing of soil sampling should be considered when soil testing for K. Researchers have found soil test K levels can vary, depending on the time of year the field was sampled. There are some patterns with K levels being lowest in fall, rising during the winter, and peaking in the early spring. Then over the growing season, K decreases down to low levels again by early fall.
Soil test K may increase over the winter as a result of K leaching from crop residues as they break down and may decrease over the growing season in response to crop uptake depleting the available supply. This may be logical, but unfortunately, soil test K doesn’t always follow the pattern expected from the mass balances of crop additions and removals. As described in Franzen et al. (2021), expectations are confounded by the potential for buffering of K removal from structural K in feldspars and from interlayer K in primary and secondary layer silicates. Similarly, surplus K additions can be fixed in interlayer positions in secondary layer silicates or in sparingly soluble neo‐formed secondary minerals.

The variability in K soil levels can be due to crop uptake, soil moisture, clay mineralogy, freeze–thaw cycles that can weather clay minerals, and microbial activity. Each of these factors can impact the amount of available K reported by a soil test.
In addition to timing, growers and agronomists should consider the importance of uniform sampling. Soils should be sampled in the same location, to the correct depth, with the proper number of samples. Soil sampling depth should follow your corresponding university or soil lab recommendations, usually 6 or 8 inches deep. The sample should be collected from the soil layers with the most intense root activity during growth stages when K uptake is critical (Bell et al., 2021). Sampling depth consistency is especially important in reduced‐tillage systems, like no‐till, where nutrients can become stratified.
The number of cores taken should also be considered to get accurate soil test K values. The number of soil cores for each sample should capture the variability of the sampling area. Keep in mind that variability in K level can be influenced by differences in landscape position, erosion, and management history. When sampling, aim to take between 8 and 12 soil cores to capture the field variability for each sample area.
Sampling strategies vary, and this brings up the topic of zone versus grid sampling. Zone sampling is designated by soil and landscape features or yield maps. In zone sampling, a composite sample should be taken for each zone. In contrast, grid sampling aims to manage fertility using a grid system where soil samples are taken from locations spread uniformly across the field.
Zone sampling has the advantage of capturing existing variability in the sampling scheme and can be more efficient in terms of samples required per acre. However, grid sampling may capture variability within zones that could be missed in the zone sample strategy. With the release of more sophisticated precision technologies to interpolate and krige, grid sampling provides the necessary sample distribution to make fertility maps. In grid or zone sampling, samples can be compared year to year if their specific field locations are recorded. There are pros and cons to each sampling philosophy, and growers should select what is most logical given their resources.
To best manage K, sampling to a uniform time and depth, taking an adequate number of cores, and returning to the same sampling locations will ensure the K values reported on your soil test can be compared between sampling years.
Factors Impacting Crop Uptake of K
While your soil might have an adequate amount of K, there are several factors that might impact K uptake in the crop. Soil moisture, temperature, and aeration can all impact crop uptake of K. Drought not only limits crop access to much‐needed water, but it also inhibits nutrient uptake. Adequate moisture in the soil is essential for K to diffuse to the plant roots. Another factor is soil aeration and oxygen. Roots need oxygen for respiration and uptake of K. There is a balance in water dynamics; too much or too little water can greatly impact K uptake and availability. Just like K reported by soil test is impacted by water dynamics, so is crop uptake. The limited mobility of K in soil implies that its uptake is limited by diffusion and depends on the extensiveness of the crop root system. Thus, crops such as cotton may have higher critical soil K levels (e.g., Bell et al., 2021), owing to their lower root length density in the soil.
Soil temperature also influences root activity and thus the plant’s access to nutrients like K. The ideal soil temperature for K uptake is between 60 and 80 °F. Tillage can also play a role in water and temperature dynamics, and in turn, influence K uptake. For no‐till soils that tend to remain cold longer into the spring, a bit of pop‐up or starter can help give those small plant roots a boost to help them access other nutrients like K (Kaiser & Rubin, 2013).
Best Practices for K Management
While clay minerals influence K availability, the tools for tailoring K recommendations to soils with different clays require more development in most places. The moist soil test may show more accurate estimates of K according to some research. The best practice is still to rely on recommendations from state extension specialists. Most K recommendations are built using dry soils and should not be used for moist soil test K values. For best management of K, follow the guidelines from your soil lab or university.
To get the most out of your soil‐testing program for K, be consistent with your sampling time, depth, number, and location. Consider both the time of year and the phase of the crop rotation. Try to uniformly sample after the same crop and in the same month each year you soil sample. This is especially important for maintaining specific soil K levels. Whether you are trying to bring your K fertility up or down, sampling at a consistent time of year will give you the best results.
It is important to sample consistently over time AND space. If grid sampling, attempt to get close to your past grid sampling location. As a rule of thumb, try to sample a 3‐m (9.8‐ft) radius area around each sampling point. Most consumer‐grade GPS systems (like those today’s smartphones) have about a 3‐m accuracy, so sampling within 3 m from a point will get you close to a consistent area of sampling each year. For those looking to fine‐tune sampling locations, adding an additional GPS receiver can help ensure accuracy, even down to the inch. Sampling in this manner allows for tracking fertility levels and accurately seeing changes due to fertility management in each location of the field.
Soil test K can be highly variable, but with consistency in soil sampling and using the recommended soil tests, growers and agronomists can determine reliable soil test K levels.
References
Barbagelata, P.A., & Mallarino, A.P. (2013). Field correlation of potassium soil test methods based on dried and field‐moist soil samples for corn and soybean. Soil Science Society of America Journal, 77(1), 318–327. https://doi.org/10.2136/sssaj2012.0253
Bell, M.J., Thompson, M.L., & Moody, P.W. (2021). Using soil tests to evaluate plant availability of potassium in soils. In T.S. Murrell, R.L. Mikkelsen, G. Sulewski, R. Norton, & M.L. Thompson (Eds.), Improving potassium recommendations for agricultural crops (pp. 191–218). Springer, Cham. https://doi.org/10.1007/978‐3‐030‐59197‐7_8
Breker, J.S., DeSutter, T., Rakkar, M.K., Chatterjee, A., Sharma, L., & Franzen, D.W. (2019). Potassium requirements for corn in North Dakota: Influence of clay mineralogy. Soil Science Society of America Journal, 83, 429–436. https://doi.org/10.2136/sssaj2018.10.0376
Franzen, D.W., Goulding, K., Mallarino, A.P., & Bell, M.J. (2021). How closely is potassium mass balance related to soil test changes? In T.S. Murrell, R.L. Mikkelsen, G. Sulewski, R. Norton, & M.L. Thompson (Eds.), Improving potassium recommendations for agricultural crops (pp. 263–282). Springer, Cham. https://doi.org/10.1007/978‐3‐030‐59197‐7_10
Kaiser, D.E., & J.C. Rubin. (2013). Corn nutrient uptake as affected by in‐furrow starter fertilizer for three soils. Agronomy Journal, 105, 1199–1210. https://doi.org/10.2134/agronj2013.0122
Mikkelsen, R.L., & T.L. Roberts. (2021). Inputs: potassium sources for agricultural systems. In T.S. Murrell, R.L. Mikkelsen, G. Sulewski, R. Norton, & M.L. Thompson (Eds.), Improving potassium recommendations for agricultural crops (pp. 47–73). Springer, Cham. https://doi.org/10.1007/978‐3‐030‐59197‐7_2
Oltmans, R.R., & Mallarino, A.P. (2015). Potassium uptake by corn and soybean, recycling to soil, and impact on soil test potassium. Soil Science Society of America Journal, 79(1), 314–327. https://doi.org/10.2136/sssaj2014.07.0272
Stucki, J.W. 1996. Potassium in the soil: Is it there or isn’t it? Some new findings that may explain its behavior. Better Crops 80(4):16‐19. http://www.ipni.net/publication/bettercrops.nsf/issue/BC‐1996‐4
Self-Study CEU Quiz
Earn 1 CEU in Nutrient Management by taking the quiz for the article at https://web.sciencesocieties.org/Learning-Center/Courses. For your convenience, the quiz is printed below. The CEU can be purchased individually, or you can access as part of your Online Classroom Subscription.
- The percentage of farm soil samples submitted to laboratories in 2020 that tested below the critical level for potassium was
- 22%.
- 44%.
- 65%.
- 75%.
- The potassium held on the soil’s cation exchange sites is considered
- unavailable.
- slowly available.
- exchangeable.
- dissolved.
- A clay type that has moderate cation exchange capacity and exhibits little shrinking and swelling upon wetting or drying is
- smectite.
- montmorillonite.
- kaolinite.
- illite.
- Soils with a higher ratio of smectite to illite have a critical soil test level for potassium that may tend to be
- higher.
- lower.
- more variable.
- less variable.
- The use of soil samples maintained at field moisture for soil K testing has potential to
- reduce costs of sampling.
- reduce lab analytical costs.
- increase accuracy.
- Increase laboratory efficiency.
- Research in Iowa showed that in soils that had been air‐dried, compared with soils maintained at field moisture, critical soil K levels were
- lower.
- the same.
- higher.
- more precise.
- In many soils, clay minerals, such as feldspars, layer silicates, and smectites, can fix or release K, leading to changes in soil test K that
- correspond to additions and removals of K.
- differ from expectations.
- provide consistent crop nourishment.
- reduce soil test K.
- Flooding conditions that lead to the chemical reduction of iron in soils with montmorillonite were observed to
- increase K fixation.
- increase K release.
- chemically reduce K.
- chemically oxidize K.
- The soil depth sampled for K should represent the
- full depth of the root zone.
- zone in which roots are most active.
- top 2 inches of soil.
- full topsoil depth.
- Consistency in soil sampling depth, timing, locations, and number of cores is important to ensure accuracy in evaluating
- trends in soil K availability over the years.
- precision of GPS location.
- sampling efficiency.
- K movement through the soil.
Text © . The authors. CC BY-NC-ND 4.0. Except where otherwise noted, images are subject to copyright. Any reuse without express permission from the copyright owner is prohibited.









